Evaluation of Distal Symmetric Polyneuropathy: The Role of Autonomic Testing, Nerve and Skin Biopsy
Justification. Polyneuropathy is a relatively common neurological disorder.10 The overall prevalence is =2,400 ( 2.4% ) per 100,000 population, but in individuals older than 55 years the prevalence rises to =8,000 ( 8% ) per 100,000.9,32 Since there are many etiologies of polyneuropathy, a logical clinical approach is needed for evaluation and management. The search yielded 1,045 references with abstracts. After reviewing titles and abstracts, 106 articles were reviewed and classified. Autonomic nervous system dysfunction occurs in several phenotypes. It may occur as one component of a generalized polyneuropathy such as DSP of diabetes. Such polyneuropathies are usually diagnosed by a combination of neuropathic symptoms, decreased or absent ankle reflexes, decreased distal sensation, distal muscle weakness or atrophy, and abnormal nerve conduction studies ( NCSs).10 The majority of these features constitute evidence of “large fiber” sensory and motor involvement. However, signs of autonomic nervous system involvement may also constitute findings indicative of DSP. In DSP with autonomic involvement, the most common clinical findings are abnormalities of sweating and circulatory instability in the feet.9,10 Currently available autonomic tests can provide indices of cardiovagal, adrenergic, and postganglionic sudomotor function. As such they provide indices for both parasympathetic and sympathetic autonomic function. Heart rate variability testing is a simple and reliable test of cardiovagal function. It detects the presence of diabetic polyneuropathy with nearly the same sensitivity as NCSs (Class II) .7 Specificity is high (97.5%) for identifying parasympathetic deficits if the recommended age-controlled values are used (Class II).26 Intrinsic cardiac disease can affect the results of this test, and this possibility must be considered in the interpretation. Autonomic testing is probably useful in documenting autonomic nervous system involvement in polyneuropathy ( Class II and III) . The sensitivity and specifi city vary with the particular test. The utilization of the combination of autonomic refl ex screening tests in the CASS provides the highest sensitivity and specifi city for documenting autonomic dysfunction ( Class II) . Autonomic testing should be considered in the evaluation of patients with polyneuropathy to document autonomic nervous system involvement ( Level B) . Autonomic testing should be considered in the evaluation of patients with suspected autonomic neuropathies ( Level B) and may be considered in the evaluation of patients with suspected distal SFSN ( Level C) . The combination of autonomic screening tests in the CASS should be considered to achieve the highest diagnostic accuracy ( Level B) . If the full battery of tests in the CASS is not available, a combination of tests of cardiovagal function ( e.g., heart rate response to deep breathing) and some test of adrenergic function may be considered as an alternative ( Level C) .24 Nerve biopsy is generally accepted as useful in the diagnosis of inflammatory diseases of nerves such as vasculitis, sarcoidosis, CIDP, infectious diseases such as leprosy, or infiltrative disorders such as tumor or amyloidosis.9 Nerve biopsy is most valuable in mononeuropathy multiplex or suspected vasculitic neuropathy. There are no studies regarding the role of nerve biopsy in the evaluation of DSP, although on occasion the above-noted diseases may present in that fashion. Out of 50 articles judged to be relevant, no article attained a grade greater than Class IV. Most of the articles discussed the nerve biopsy findings in specific diseases, the clinical suspicion of which had prompted the biopsy.1– 3,5,6,13,14,34,39,40–42 No article provided guidance regarding when to perform a nerve biopsy in the evaluation of DSP. There is no evidence to support or refute a conclusion regarding the role of nerve biopsy in the evaluation of DSP ( Class IV ). No recommendations can be made regarding the role of nerve biopsy in determining the etiology of DSP ( Level U). Skin biopsy is being increasingly used to evaluate patients with polyneuropathy. The most common techniq ue involves a 3-mm punch biopsy of skin from the leg. After sectioning by microtome, the tissue is immunostained with anti-protein-gene-product 9.5 ( PG P 9.5) antibodies and examined with immunohistochemical or immunofl uorescent methods. This staining allows for the identification and counting of intraepidermal nerve fi bers ( IE NF) . PG P 9.5 immunohistochemistry has been validated as a reliable method for IE NF density determination with good intra- and interobserver reliability in normal controls and patients with DSP.15,20,33,48 Beyond distinguishing asymptomatic normals from polyneuropathy patients, one clinical question not addressed by the EFNS guideline was the diagnostic accuracy of skin biopsy in distinguishing symptomatic patients with polyneuropathy from symptomatic patients without polyneuropathy. For example, in patients with painful feet, would skin biopsy accurately distinguish patients with polyneuropathy from patients with other conditions causing painful feet? To address this separate question, a subgroup of the Polyneuropathy Task Force ( J.D.E ., R.A.L., D.H., G.L., M.P., and G.S.G .) independently reviewed the literature regarding the diagnostic accuracy of skin biopsy in DSP and in the SFSN form of DSP. To be considered for review, studies needed to determine IENF density in patients with and without polyneuropathy. Furthermore, the data from studies had to be presented in such a way as to allow calculation of the sensitivity and specifi city of skin biopsy for polyneuropathy. IENF density assessment using PGP 9.5 immunohistochemistry is a validated, reproducible marker of small fiber sensory pathology. Skin biopsy with IENF density assessment is possibly useful to identify DSP that includes SFSN in symptomatic patients with suspected polyneuropathy. (Class III). For symptomatic patients with suspected polyneuropathy, skin biopsy may be considered to diagnose the presence of a polyneuropathy, particularly SFSN (Level C).
This comprehensive review reveals several weaknesses in the current approach to the evaluation of polyneuropathy and highlights opportunities for research.
The AAN, the AANEM, and the AAPM&R determined that there was a need for an evidence-based and clinically relevant practice parameter for the evaluation of polyneuropathy. As a prelude to this project, the three organizations developed a formal case definition of distal symmetric polyneuropathy DSP.10 As outlined in this previous publication, the most accurate diagnosis of distal symmetric polyneuropathy is provided by a combination of neuropathic symptoms, signs, and electrodiagnostic (EDX) studies. Since EDX studies are sensitive, specific, and validated measures of the presence of polyneuropathy and can distinguish between demyelinating and axonal types of neuropathy, they should be included as an integral part of the diagnosis.10 This practice parameter assumes that a clinical diagnosis of polyneuropathy has been determined based on such criteria. The diagnosis and evaluation of polyneuropathy is complex. The practice parameter is not intended to replace the clinical judgment of experienced physicians in the evaluation of polyneuropathy. The particular kinds of tests utilized by a physician in the evaluation of polyneuropathy depend on the specific clinical situation and the informed medical judgment of the treating physician. The AAN, AANEM, and AAPM&R are committed to producing independent, critical, and truthful clinical practice guidelines (CPGs) . Significant efforts are made to minimize the potential for conflicts of interest to influence the recommendations of this CPG. To the extent possible, the AAN, AANEM, and AAPM&R keep separate those who have a financial stake in the success or failure of the products appraised in the CPGs and the developers of the guidelines. Conflict of interest forms were obtained from all authors and reviewed by an oversight committee prior to project initiation. AAN, AANEM, and AAPM&R limit the participation of authors with substantial conflicts of interest. The AAN, AANEM, AAPM&R forbid commercial participation in, or funding of, guideline projects. Drafts of the guideline have been reviewed by at least three AAN committees, AANEM and AAPM&R committees, a network of neurologists, Neurology peer reviewers, and representatives from related fields. The AAN Guideline Author Conflict of Interest Policy can be viewed at www.aan.com. Quality Standards Subcommittee (AAN): Jacqueline French, MD, FAAN ( co-chair) ; Gary S. Gronseth, MD ( co-chair) ; Charles E . Argoff, MD; Eric Ashman, MD; Stephen Ashwal, MD, FAAN ( ex-officio) ; Christopher Bever Jr., MD, MBA, FAAN; John D. England, MD, FAAN ( Q SS facilitator) ; Gary M. Franklin, MD, MPH, FAAN ( ex-officio) ; Deborah Hirtz, MD ( ex-officio) ; Robert G . Holloway, MD, MPH, FAAN; Donald J. Iverson, MD, FAAN; Steven R. Messe´ , MD; Leslie A. Morrison, MD; Pushpa Narayanaswami, MD, MBBS; James C. Stevens, MD, FAAN ( ex-officio) David J. Thurman, MD, MPH ( ex-officio); Samuel Wiebe, MD; Dean M. Wingerchuk, MD, MSc, FRCP (C) ; and Theresa A. Zesiewicz, MD, FAAN. Practice Issues Review Panel (AANEM): Yuen T. So, MD, PhD ( chair) ; Michael T. Andary, MD; Atul Patel, MD; Carmel Armon, MD; David del Toro, MD; Earl J. Craig, MD; James F. Howard, MD; Joseph V. Campellone Jr., MD; Kenneth James Gaines, MD; Robert Werner, MD; Richard Dubinsky, MD.
Practice Guidelines Committee (AAPM& R): Dexanne B. Clohan, MD ( chair) ; William L. Bockenek, MD; Lynn Gerber, MD; Edwin Hanada, MD; Ariz R. Mehta, MD; Frank J. Salvi, MD, MS; and Richard D. Zorowitz, MD. Classification of Evidence for Studies of Diagnostic Accuracy: Class I: Evidence provided by a prospective study in a broad spectrum of persons with the suspected condition, using a “gold standard” for case definition, where a test is applied in a blinded evaluation, and enabling the assessment of appropriate tests of diagnostic accuracy. Classification of Recommendations:
INTRODUCTION
This practice parameter provides recommendations for the evaluation of distal symmetric polyneuropathy ( DSP) based on a prescribed review and analysis of the peer-reviewed literature. The parameter was developed to provide physicians with evidence-based guidelines regarding the role of autonomic testing, nerve biopsy, and skin biopsy for the assessment of polyneuropathy. The diagnosis of DSP should be based on a combination of clinical symptoms, signs, and electrodiagnostic criteria as outlined in the previous case defi nition.10 (See Mission Statement, below, for details.)
Formation of Expert Panel. The Polyneuropathy Task Force included 19 physicians with representatives from the American Academy of Neurology (AAN) , the American Academy of Neuromuscular and Electrodiagnostic Medicine (AANEM) , and the American Academy of Physical Medicine and Rehabilitation (AAPM&R) . All of the task force members had extensive experience and expertise in the area of polyneuropathy. Additionally, four members had expertise in evidence-based methodology and practice parameter development. Three are current members (J.D.E., G.S.G., G.F.), and one is a former member (R.G.M.) of the Quality Standards Subcommittee (QSS) of the AAN. The task force developed a set of clinical questions relevant to the evaluation of DSP, and subcommittees were formed to address each of these questions.DESCRIPTION OF THE ANALYTIC PROCESS
Panel experts were asked to identify additional articles missed by the initial search strategy. Further, the bibliographies of the selected articles were reviewed for potentially relevant articles.
Subgroups of committee members reviewed the titles and abstracts of citations identified from the original searches and selected those that were potentially relevant to the evaluation of polyneuropathy. Articles deemed potentially relevant by any panel member were also obtained.
Each potentially relevant article was subsequently reviewed in entirety by at least three panel members. Each reviewer graded the risk of bias in each article by using the diagnostic test classification-of-evidence scheme (Appendix 2) . In this scheme, articles attaining a grade of Class I are judged to have the lowest risk of bias, and articles attaining a grade of Class IV are judged to have the highest risk of bias. Disagreements among reviewers regarding an article’s grade were resolved through discussion. Final approval was determined by the entire panel.
The Quality Standards Subcommittee (AAN), the Practice Issues Review Panel (AANEM), and the Practice Guidelines Committee (AAPM&R) (Appendix 1A–C) reviewed and approved a draft of the article. The draft was next sent to members of the AAN, AANEM, and AAPM&R for further review and then to Neurology for peer review. Boards of the AAN, AANEM, and AAPM&R reviewed and approved the final version of the article. At each step of the review process, external reviewers’ suggestions were explicitly considered. When appropriate, the expert panel made changes to the document.
ANALYSIS OF THE EVIDENCE
Role of Clinical Autonomic Testing in the Evaluation of Polyneuropathy.
A second phenotype is that of an autonomic neuropathy such as in amyloidosis and autoimmune autonomic neuropathy, where autonomic nerves are affected disproportionately relative to somatic nerves.29 In these neuropathies, autonomic fibers can be affected in isolation and their involvement may precede somatic fiber involvement.47
A third relatively common phenotype is distal small fiber sensory polyneuropathy ( SFSN), which can manifest as burning pain affecting the feet, often with allodynia and sometimes with erythromelalgia (red hot and painful skin). Involvement of autonomic and somatic C fibers usually occurs concurrently in small fiber polyneuropathy.47What Is the Usefulness of Clinical Autonomic Testing in the Evaluation of Polyneuropathy, and Which Tests Have the Highest Sensitivity and Specificity?
Cardiovagal function can be evaluated using different indices in the time and frequency domains.56 There is no compelling evidence that one method is better than another or that the use of multiple indices confers any advantage. Heart rate variability to deep breathing is the most widely used test of cardiovagal function and has a specificity of =80% ( Class II).24
The vagal component of the baroreflex can be evaluated by quantitating the heart period response to induced changes in blood pressure ( BP). A well studied test is the modified Oxford method.8 The test consists of an evaluation of heart period responses to induced increases and decreases in arterial BP. The increase is evoked by intravenous phenylephrine and decrease by nitroprusside in incremental doses. Baroreflex sensitivity is defined by the slope of the heart period to BP relationship. Linearity is required (R" 0.85). The advantage of this test is that it evaluates vagal baroreflex sensitivity; however, the disadvantage is that the test is invasive and not widely performed. Approximation of this method is possible by relating heart period alterations to changes in BP induced by the Valsalva maneuver.52 The sensitivity and specificity of invasive and noninvasive tests of baroreflex function are high, but these tests are not generally used in the study of neuropathy since their value is considered only additive to current tests of cardiovagal function (Class II).24,29,44,54
Thermoregulatory sweat testing (TST) is a sensitive test of sudomotor function that utilizes an indicator substance whose color changes upon exposure to sweat.12,30 The test results can be semiquantitated by estimating the percentage of skin surface that is anhidrotic. Since the test is tedious, messy, and time consuming, it is not routinely done. Additionally, TST is not able to distinguish between postganglionic, preganglionic, and central lesions.12,30 The most quantitative test of sudomotor function is the quantitative sudomotor axon reflex test (QSART).25 QSART is mediated by impulses traveling antidromically then orthodromically along the postganglionic sympathetic sudomotor axon. QSART can detect distal sudomotor loss with a sensitivity of 75% – 90% (Class III).26,35,50,51 Several studies have demonstrated that QSART can determine sudomotor abnormalities with relatively high sensitivity and specificity in many types of polyneuropathies (Class II and III).7,25,26,28,29,31,35,47,50 In three Class III studies, QSART was shown capable of detecting distal small fiber polyneuropathy with a sensitivity of "75% .35,50,51
Skin vasomotor reflexes assessed by monitoring skin blood flow using laser Doppler flowmeter has not been well studied. Limited data from one Class III study using this technique demonstrated an unacceptably large coefficient of variation.27
Analysis of the available Class II and III studies on autonomic testing indicate that a combination of autonomic reflex screening tests provides distinct advantages over single modality methods. The composite autonomic scoring scale ( CASS) , which includes QSART, orthostatic blood pressure, heart rate response to tilt, heart rate response to deep breathing, the Valsalva ratio, and beat-to-beat BP measurements during Phases II and IV of the Valsalva maneuver, tilt, and deep breathing provides a useful 10-point scale of autonomic function ( Class II) .24,29 In a study of 78 patients with graded autonomic failure obtained by selecting approximately equal numbers of patients with multiple system atrophy, Parkinson’s disease, autonomic neuropathies, and idiopathic peripheral neuropathies, this combination of tests provided a noninvasive, sensitive, specific, and reproducible methodology for grading the degree of autonomic dysfunction ( Class II) .24Conclusions.
Recommendations.
Role of Nerve Biopsy in the Evaluation of Polyneuropathy.
What Is the Usefulness of Nerve Biopsy in Determining the Etiology of Distal Symmetric Polyneuropathy?
Conclusions.
Recommendations.
Role of Skin Biopsy in the Evaluation of Polyneuropathy.
In March 2005 the European Federation of Neurological Societies ( E FNS) published a guideline on the use of skin biopsy in peripheral neuropathy.20 This comprehensive review focused on the technical aspects of skin biopsy as well as normative data and correlations with other clinical, physiologic, and pathologic tools. The EFNS concluded that skin biopsy is a safe, validated, and reliable technique for the determination of IENF density. The major conclusion was that skin biopsy (IENF density) was diagnostically efficient at distinguishing polyneuropathy patients (including small fiber neuropathy) from normal controls. The EFNS guideline also reviewed the literature on IENF morphologic changes such as axonal swellings as a measure of distal symmetric polyneuropathy.16,20,21 The EFNS concluded that axonal swellings may be predictive of progression of polyneuropathy but further studies were needed to determine their diagnostic accuracy.20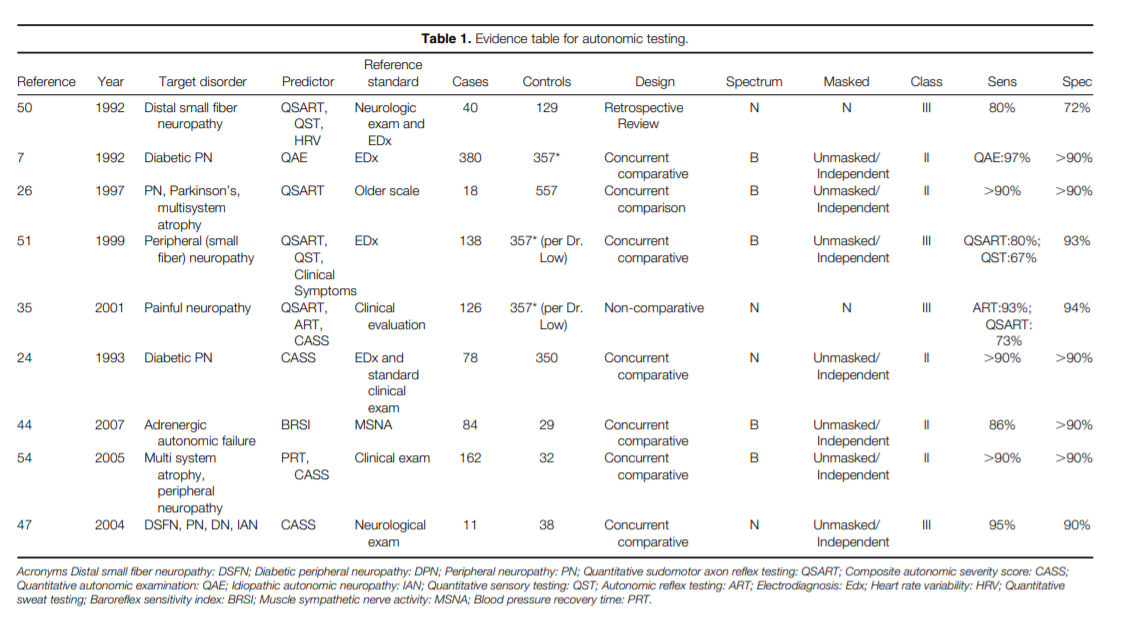
What Is the Usefulness and Diagnostic Accuracy of Skin Biopsy in the Evaluation of Polyneuropathy?
Nine studies met inclusion criteria.4,16– 18,21–22,33,36– 37 One was a prospective cohort survey of patients presenting with bilateral painful feet and normal strength, but skin biopsy was done only in those with normal NCS.36 Patients with reduced IENF density and normal NCS were assumed to have painful small fi ber neuropathies. However, the study did not compare the results of the IENF density to an independent reference standard to confirm the presence of small fiber neuropathy. Thus, for the purposes of determining the diagnostic accuracy of skin biopsy for polyneuropathy, this study was graded Class IV.
The remaining studies employed a case-control design.16,18,21,22,33 In these studies the investigators performed skin biopsies on patients with established polyneuropathy and normal controls. No study included patients with conditions causing lower extremity pain or sensory complaints that might be confused with polyneuropathy. Thus, all studies had potential spectrum bias. Following the evidence classifi cation scheme for studies of diagnostic accuracy, all of these studies were graded Class III.
All of the case-control studies showed a significant reduction in IENF density in polyneuropathy patients as compared to controls.16,18,21,22,33 The sensitivity of decreased IENF density for the diagnosis of polyneuropathy was moderate to good (range 45% – 90%) . The specificity of normal IENF density for the absence of polyneuropathy was very good (range 95% – 97%) . Thus, the absence of reduced IENF density (using the clinical impression as the diagnostic reference standard) would not “rule out” polyneuropathy, but the presence of reduced IEFN density would importantly raise the likelihood of polyneuropathy.
The form of DSP for which IENF assessment is particularly diagnostically attractive is SFSN for the following reasons: (1) IENF are the nerve terminals of somatic unmyelinated C fibers, which are hypothesized to be predominantly affected in SFSN; (2) There has been a lack of a direct objective measure of small fiber sensory nerves since objective measures of large fiber function (e.g., NCS) are by most definitions normal in SFSN19; 3) Patients in whom SFSN is clinically suspected manifest with symptoms of small fiber sensory dysfunction (e.g., tingling, numbness, and neuropathic pain) but few objective signs, making it difficult to diagnose and to distinguish SFSN from nonneurological causes of sensory complaints.19
Since no validated objective gold standard exists for the diagnosis of SFSN, the authors considered whether demonstration of a pathologiclesion (small sensory fiber pathology on skin biopsy) should be the defacto diagnostic standard or whether a clinical impression of SFSN should be the independent reference standard. For the purposes of this parameter, a clinical impression of SFSN was adopted as the independent reference standard for calculation of sensitivity and specifi city of IENF density in the detection of SFSN.
In order to assess the diagnostic accuracy of IENF density assessment for SFSN, the literature was surveyed for studies assessing IENF density in subjects with clinically suspected SFSN (symptoms or symptoms and signs of DSP but with normal NCS) and controls where the diagnostic accuracy of IENF density for clinically defined SFSN could be determined. Four Class III studies met these criteria.18,23,46,55 The sensitivity of IENF density assessment at the ankle for DSP with normal NCS was 58% (20% for subjects with symptoms but no signs of SFSN; 100% for subjects with symptoms and signs of SFSN) ,46 90% ,18 and 24% .23 In these studies, the specificity of the test ranged from 95% – 97.5% .18,23,46 The other case-control study found that among patients with symptoms of SFSN and an abnormal pinprick examination in the feet, but normal ankle refl exes, normal vibration sensibility, and normal NCS that an IE NF density of #8 fibers/mm at the dorsal foot provided a sensitivity of 88% , a specificity of 91% , a positive predictive value of 0.9, and a negative predictive value of 0.83 for the diagnosis of SFSN.55Conclusions.
Recommendations.
RECOMMENDATIONS FOR FUTURE RESEARCH
Mission Statement.
Disclaimer.
This statement is provided as an educational service of the AAN, AANEM, and the AAPM&R. It is based on an assessment of current scientific and clinical information. It is not intended to include all possible proper methods of care for a particular neurologic problem or all legitimate criteria for choosing to use a specific test or procedure. Neither is it intended to exclude any reasonable alternative methodologies. The AAN, AANEM, and AAPM&R recognize that specific care decisions are the prerogative of the patient and physician caring for the patient, based on all of the circumstances involved.Conflict of Interest.
APPENDIX 1 A
APPENDIX 1 B
APPENDIX 1 CAPPENDIX 2
Class II: Evidence provided by a prospective study of a narrow spectrum of persons with the suspected condition, or a well-designed retrospective study of a broad spectrum of persons with an established condition ( by “gold standard”) compared to a broad spectrum of controls, where a test is applied in a blinded evaluation, and enabling the assessment of appropriate tests of diagnostic accuracy.
Class III: Evidence provided by a retrospective study when either persons with the established condition or controls are of a narrow spectrum, and where a test is applied in a blinded evaluation.
Class IV: Any design where a test is not applied in blinded evaluation or evidence provided by expert opinion alone or in descriptive case series ( without controls).APPENDIX 3
A= Established as effective, ineffective, or harmful for the given condition in the specified population. (Level A rating requires as least two consistent Class I studies.) B= Probably effective, ineffective, or harmful for the given condition in the specified population. (Level B rating requires at least one Class I study or at least two consistent Class II studies.) C= Possibly effective, ineffective, or harmful for the given condition in the specified population. ( Level C rating requires at least one Class II study or two consistent Class III studies.) U= Data inadequate or conflicting; given current knowledge, treatment is unproven.
With regard to conflicts of interest, the authors disclose the following: (1) Holds financial interests in Pfizer. (2) Holds financial interests in Pfizer and GlaxoSmithKline and Boeheringer Ingelheim for speaker honoraria and Ortho-McNeil for serving on the IDMC Committee. (3) Nothing to disclose. (4) Nothing to disclose. (5) Received royalties from the American Medical Resources, Enduring Medical Materials (CD/ DVD) , has received honorarium from Medical Education Resources, CME LLC, E xpert Witness testimony and record review, Peters Marketing Research, Delve Marketing Research, Cross Country Education and American Medical Seminars. Dr. Kinsella holds corporate appointments with Cross Country Education and Forest Park Hospital. (6) Nothing to disclose. (7) Receives residual royalties from
Elsevier for editorial work done prior to 2005. He receives honoraria from the Dana Foundation, NY, and the International Society for Neuroimmunology. His wife is a consultant for the Dana Foundation. (8) Nothing to disclose. (9) Financial interests in Athena Diagnostics and has received research funding from NIH/NEI, NIH/ NIDCR, Charcot-Marie-Tooth Association, and the March of Dimes. (10) Serves as a Scientific Advisor for Quest Diagnostics and is a member of a Steering Committee, Talecris Biotherapeutics. Dr. Latov receives royalties from Demos publications and has received research support from the NIH and Talecris Biotherapeutics. He holds stock options in Therapath LLC and is the beneficiary of license fee payments from Athena Diagnostics to Columbia University. He has given expert testimony in legal proceedings related to neuropathy and has prepared anaffidavit with regarding to the legal proceeding related to neuropathy. (11) Financial interests in Talecris and has received research funding from MDA and CMTA. He estimates that approximately 33% of his clinical effort is spent on electromyography. He has received payment for expert testimony regarding the use of IVIg in CIDP; neuropathic pain after breast reduction. (12) Served as a consultant for WR Medical, Viatris, Eli Lilly and Company, Chelsea Therapeutics, and Quigley Corporation. (13) Financial interests in Astrazeneca, Photothera, Wyeth, Jalmarjone Sahron, Inarx, Boehringer-Ingelheim, Dullehi-Arubio, Axaron, U-Servicer, and PAION. (14) Estimates that approximately 15% – 20% of his clinical effort is spent on skin biopsies. (15) Serves on a myasthenia gravis medical scientific board, has served as an Associate Editor, Journal of Clinical Neuromuscular Disease (1998– 2006) , receives honoraria from Duke University Medical Center, and Medical Educational Resources. He is the director of MEG laboratories and estimates that 75% of his time is spent there. He also holds stock options in GE, Pfizer, and Johnson & Johnson. In addition, he has provided an affidavit on two cases regarding myasthenia gravis. (16) Financial interests in GlaxoSmithKline and Formenti-Grunenthal. In addition he has received research funding from Pfitzer, Formenti-Grunenthal, Foramenti-G runenthal, Italian Ministry of Health, and Regione Lombardia. (17) Financial interests in Celgene and Pathologica. (18) Financial interests in DSMB, Pfizer, Johnson & Johnson, Mitsubishi Pharma, Merck, Xenoport, and GSK. He has received research funding from JDRF, NIH, Astellas Pharma, Mitsubishi Pharma, and Sanofi -Aventis. He estimates that 10% of his clinical effort is devoted to EMG, 5% to skin biopsy, and #1% on lumbar puncture. (19) Received payment for expert testimony in the possible neurotoxic injury of the peripheral nerve.
References
[ Note. Strength of evidence is indicated for references used to formulate conclusions and recommendations.]
- Argov Z , Nagle D, G iger U, Leigh JS Jr. The yield of sural nerve biopsy in the evaluation of peripheral neuropathies. Acta Neurol Scand 1989;79:243– 245.
- Bosboom WM, V an den Berg LH, Franssen H, G iesbergen PC, Flach HZ , van Putten AM, et al. Diagnostic value of sural nerve demyelination in chronic demyelinating polyneuropathy. Brain 2001;124:2427– 2438.
- Chia L, Fernandez A, Lacroix C, Adams D, Plante´ V , Said G . Contribution of the nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly: a retrospective review of 100 consecutive patients. Brain 1996;119:1091– 1098.
- Chien HF, Tseng TJ, Lin WM, Yang CC, Chang YC, Chen RC, et al. Quantitative pathology of cutaneous nerve terminal degeneration in the human skin. Acta Neuropathol 2001;102: 455– 461. ( Class III)
- Deprez M, Ceuterick-DeG roote C. Clinical and neuropathological parameters affecting the diagnostic yield of nerve biopsy. Neuromusc Disorders 2000;10:92– 98.
- Deprez M, Ceuterick-de G roote C, Schoenen J, Reznik M, Martin JJ. Nerve biopsy: indications and contribution of the diagnosis of peripheral neuropathy. Acta Neurol Belg 2000; 100:162– 166.
- Dyck PJ, Karnes JL, O ’Brien PC, Litchy WJ, Low PA, Melton LJ. The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology 1992;42:1164– 1170. ( Class II)
- Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol 1992; 263:798– 803.
- England JD, Asbury AK. Peripheral neuropathy. Lancet 2004; 363:2151– 2161.
- England JD, Gronseth G S, Franklin G , Miller RG , Asbury AK, Carter G T, et al. Distal symmetric polyneuropathy: a definition for clinical research. Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199– 207.
- Facer P, Mann D, Mathur R, Pandya S, Ladiwala U, Singhal B, et al. Do nerve growth factor-related mechanisms contribute to loss of cutaneous nociception in leprosy? Pain 2000;85: 231– 238.
- Fealey RD, Low PA, Thomas JE . Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc 1989;64: 617– 628.
- Flachenecker P, Janka M, G oldbrunner R, Toyka KV . Clinical outcome of sural nerve biopsy: a retrospective study. J Neurol 1999;246:93– 96.
- Gabriel CM, Howard R, et al. Prospective study of the usefulness of sural nerve biopsy. J Neurol Neurosurg Psychiatry 2000;69:442– 446.
- Goranson LG , Mellgren SI, Lindal S, Omdal R. The effect of age and gender on epidermal nerve fiber density. Neurology 2004;62:774– 777.
- Herrmann DN, McDermott MP, Henderson D, Chen L, Akowuah K, Schifi tto G , and The North East Aids Dementia ( Nead) Consortium. Epidermal nerve fiber density, axonal swellings and Q ST as predictors of HIV distal sensory neuropathy. Muscle Nerve 2004;29:420– 427. ( Class III)
- Kennedy WR, Wendelschafer-Crabb G , Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology 1996;47:1042– 1048. ( Class III)
- Koskinen M, Hietaharju A, Kylaniemi M, Peltola J, Rantala I, Udd B, et al. A quantitative method for the assessment of intraepidermal nerve fibers in small-fiber neuropathy. J Neurol 2005;252:789– 794. ( Class III)
- Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002;26: 173– 188.
- Lauria G , Cornblath DR, Johansson O , McArthur JC, Mellgren SI, Nolano M, et al. E FNS Guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:1– 12.
- Lauria G , Morbin M, Lombardi R, et al. Axonal swellings predict the degeneration of epidermal nerve fi bers in painful neuropathies. Neurology 2003;61:631– 636. ( Class III)
- Li J, Bai Y, G handour K, et al. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain 2005;128:1168– 1177. ( Class III)
- Loseth S, Lindal S, Stalberg E , Mellgren SI. Intraepidermal nerve fibre density, quantitative sensory testing and nerve conduction studies in a patient material with symptoms and signs of sensory polyneuropathy. E ur J Neurol 2006;13:105–111. ( Class III)
- Low PA. Composite Autonomic Scoring Scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993;68:748– 752. ( Class II)
- Low PA, Caskey PE , Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol 1983;14:573– 580.
- Low PA, Denq JC, O pfer-G ehrking TL, Dyck PJ, O ’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997;20:1561– 1568. ( Class II)
- Low PA, Neumann C, Dyck PJ, Fealey RD, Tuck RR. E valuation of skin vasomotor reflexes by using laser Doppler velocimetry. Mayo Clin Proc 1983;58:583– 592. ( Class III)
- Low PA, Opfer-Gehrking TL, Proper CJ, Zimmerman I. The effect of aging on cardiac autonomic and postganglionic sudomotor function. Muscle Nerve 1990;13:152– 157.
- Low PA, Vernino S, Suarez G . Autonomic dysfunction in peripheral nerve disease. Muscle Nerve 2003;27:646– 661.
- Low PA, Walsh JC, Huang C, McLeod JG . The sympathetic nervous system in diabetic neuropathy: a clinical and pathological study. Brain 1975;98:341– 356.
- Low PA, Zimmerman BR, Dyck PJ. Comparison of distal sympathetic with vagal function in diabetic neuropathy. Muscle Nerve 1986;9:592– 596.
- Martyn CN, Hughes RAC. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry 1997;62:310– 318.
- McArthur JC, Stocks E A, Hauer P, Cornblath DR, G riffi n JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol 1998;55:1513– 1520. ( Class III)
- Molenacv DSM, Vermeulen M, et al. Diagnostic value of sural nerve biopsy in chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 1998;64:84– 89.
- Novak V , Freimer ML, Kissel JT, et al. Autonomic impairment in painful neuropathy. Neurology 2001;56:861– 868. (Class III)
- Periquet MI, Novak V , Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 1999;53:1641– 1647. ( Class IV )
- Pittenger G L, Ray M, Burcus NI, et al. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care 2004;27:1974– 1979. ( Class III)
- Polydefkis M, Sirdofsky M, Hauer P, Petty BG , Murinson B, McArthur JC. Factors influencing nerve regeneration in a trial of timcodar dimesylate. Neurology 2006;66:259– 261.
- Rappaport WD, Valente J, et al. Clinical utilization and complications of sural nerve biopsy. Am J Surg 1993;166:252– 256.
- Said G . Indications and usefulness of nerve biopsy. Arch Neurol 2002;59:1532– 1535.
- Said G . Indications and value of nerve biopsy. Muscle Nerve 1999;22:1617– 1619.
- Said G . Value of nerve biopsy. Lancet 2001;357:1220– 1221.
- Schiffmann R, Hauer P, Freeman B, et al. Enzyme replacement therapy and intraepidermal innervation density in Fabry disease. Muscle Nerve 2006;34:53– 56.
- Schrezenmaier C, Singer W, Muenter-Swift N, Sletten D, Tanabe J, Low PA. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol 2007;64:381– 386. ( Class II)
- Scott LJ, Griffin JW, Luciano C, et al. Quantitative analysis of epidermal innervation in Fabry disease. Neurology 1999;52: 1249– 1254.
- Shun CT, Chang YC, Wu HP, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 2004;127:1593– 1605. ( Class III)
- Singer W, Spies JM, McArthur, et al. Prospective evaluation of somatic and autonomic small fibers in selected neuropathies. Neurology 2004;62:612– 618. ( Class III)
- Smith AG , Howard JR, Kroll R, Ramchandran P, Hauer P, Singleton JR, et al. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci 2005; 228:65– 69.
- Sousa MM, Ferrao J, Fernandes R, et al. Deposition and passage of transthyretin through the blood-nerve barrier in recipients of familial amyloid polyneuropathy livers. Lab Invest 2004;84:865– 873.
- Stewart AG , Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992;15:661– 665. ( Class III)
- Tobin K, Guliani MJ, LaComis D. Comparison of different modalities for detection of small fiber neuropathy. Clin Neurophysiol 1999;110:1909– 1912. ( Class III)
- Trimarco B, Volpe M, Ricciardelli B, Vigorito C, de Luca N, Sacca L, et al. Valsalva maneuver in the assessment of baroreflex responsiveness in borderline hypertensives. Cardiology 1983;70:6– 14.
- Tseng MT, Hsieh SC, Shun CT, et al. Skin denervation and cutaneous vasculitis in systemic lupus erythematosus. Brain 2006;129:977– 985.
- Vogel E R, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 2005;65:1533– 1537. ( Class II)
- Walk D, Wendelschafer-Crabb G , Davey C, Kennedy WR. Concordance between epidermal nerve fiber density and sensory examination in patients with symptoms of idiopathic small fiber neuropathy. J Neurol Sci 2007;255:23– 26. ( Class III)
- Ziegler D, Dannehl K, Muhlen, Spuler M, Gries FA. Presence of cardiovascular autonomic dysfunction assessed by spectral analysis, and standard tests of heart rate variation and blood pressure response at various stages of diabetic neuropathy. Diabet Med 1992;9:806– 814.
Document History
Creation of New Guidelines, Consensus Statements, or Position Papers
AANEM members are encouraged to submit ideas for papers that can improve the understanding of the field. The AANEM will review nominated topics on the basis of the following criteria:- Members’ needs
- Prevalence of condition
- Health impact of condition for the individual and others
- Socioeconomic impact
- Extent of practice variation
- Quality of available evidence
- External constraints on practice
- Urgency for evaluation of new practice technology
