Establishing High-Quality Reference Values for NCS: From the Normative Data Task Force of AANEM
TIMOTHY DILLINGHAM MD,1 SHAN CHEN MD, PhD,2 MICHAEL ANDARY MD,3 RALPH BUSCHBACHER MD,4 DAVID DEL TORO MD,5 BENN SMITH MD,6 KUNO ZIMMERMANN DO, PhD,7 and YUEN SO MD8
- Department of Physical Medicine and Rehabilitation, University of Pennsylvania, 1800 Lombard Street, First Floor, Philadelphia, Pennsylvania 19146, USA
- Department of Neurology, Rutgers, the State University of New Jersey, Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA
- Department of Physical Medicine and Rehabilitation, College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan, USA
- Department of Physical Medicine and Rehabilitation, Indiana University, Indianapolis, Indiana, USA
- Department of Physical Medicine and Rehabilitation, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
- Department of Neurology, Mayo Clinic, Scottsdale, Arizona, USA
- Qinqunxx Institute, Rosharon, Texas, USA
- Department of Neurology, Stanford University, Stanford, California, USA
ABSTRACT: Introduction: There are not uniform standards for nerve conduction testing across the United States. The objective of this study is to present a set of methodologically sound criteria to evaluate the literature for the purpose of identifying high-quality normative nerve conduction studies (NCS) suitable for widespread use. Methods: The Normative Data Task Force (NDTF) was formed to review published studies on methodological issues related to NCS. A set of criteria was then developed to evaluate the literature. These criteria and their rationale are described. Results: We identified 7 key issues that reflect high quality in NCS. For each issue, specific review criteria were developed. Conclusion: Rigorous criteria enable identification of high-quality studies dealing with nerve conduction reference values. This represents the first step toward the overarching goal of recommending NCS techniques and reference values for electrodiagnostic medicine.
Muscle Nerve 54: 366–370, 2016
Electrodiagnostic (EDX) medicine has been in existence since the 1940s. EDX physicians and technologists perform nerve conduction studies (NCS) and needle electromyography to evaluate disorders of the peripheral nervous and musculoskeletal systems. In the past few decades, EDX instrumentation and signal processing have benefitted greatly from technological advancements in computing and electronics.
The American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) has been involved in standardizing how the specialty is practiced through publication of guidelines and practice parameters.1 The recent accreditation of EDX laboratories is one step in standardizing and defining quality care across the field. However, there is no universal standard for NCS in the United States.2 Individual laboratories have historically been encouraged to derive their own reference values despite inherent methodological and statistical challenges with this approach. The majority of EDX physicians and laboratories, rather than develop their own reference values, instead rely on the peer reviewed literature, textbooks, or reference values generated by academic training programs. However, many published studies do not meet contemporary standards for statistical and methodological rigor.3,4
Performing NCS on nerves and deciding whether the results fall within an expected range of normal values with a statistical level of precision is fundamental to EDX medicine. Lack of NCS standardization diminishes reliability and consistency in identification of pathology.
Such concerns were demonstrated in a recent study that assessed intra- and interobserver reliability when patients with diabetic sensorimotor polyneuropathy were evaluated.5 There was a 37.5% difference in the interobserver judgment of individual nerves as being normal, which was attributed to variability in the performance of NCS and in the use of varied reference values. This variability was of sufficient magnitude to be a concern for therapeutic trials. One recommendation to improve interrater reliability in therapeutic trials was strict standardization of NCS procedures.5 In a companion study by the same research group, institution of standard instructions, techniques, procedures, and reference values led to better interobserver judgment of abnormalities.6
Table 1. Normative Data Task Force criteria for evaluating an article for consideration as a normative standard
|
The AANEM formed the Normative Data Task Force (NDTF) in 2011 to examine how NCS are performed and to assess the scientific underpinnings of these clinical techniques, with the goal of finding the best reference values for clinical nerve conduction testing.
The initial charge for the NDTF was to derive a framework for systematically evaluating the published literature. The rationale, criteria, and methodology for assessing the published literature are presented here.
RESULTS
The NDTF evaluated published methodological studies relevant to conducting NCS and deriving reference values. These studies and the insights from NDTF members led to the development of 7 criteria (Table 1). The rationale for each criterion is described below and provides the framework for systematic evaluation of the NCS literature.Year Published. Because of differences between older analog EMG machines and modern computer-based digital equipment, the NDTF focused on works published after 1990, which more likely reflect studies conducted using modern equipment. Literature searches used the terms “nerve conduction” or “nerve conduction studies” and the name or names of the nerves under investigation in the following databases: PubMed/Medline; EMBASE; Web of Science; and Scopus.
Subject Sample Size. Large sample sizes increase power and precision and are important for normative data studies, because the upper or lower limits of the underlying distribution are significantly influenced by extreme values (outliers). Large sample sizes ensure representation of the distribution and allow for the determination of specific percentile values at the distribution extremes. Large sample sizes also represent more accurately the population under investigation. A minimum sample size is based on the expected variability of the parameters analyzed and the number of covariates in the analyses. The literature provides limited direction regarding proper sample sizes necessary for EDX study design.7 Many published EDX studies have had fewer than 50 subjects.3,4
Chang et al.8 used statistical modeling and empirical validations to examine sample sizes and their relationships to mean square errors (MSEs). As sample sizes increased from 20 to 50 subjects, MSEs were reduced by 66%; with a sample size of 100, MSEs decreased by 80%. These results suggest that a sample of at least 100 is necessary to derive normative data with reduced bias and greater precision. Other researchers suggest sample sizes of no less than 100 when demarcating the 2.5% cut-off,9 and Falck et al.10 suggested that samples of up to 300 may be optimal. The sample size must relate to the degree of expected variability of a nerve conduction parameter. Based on these studies, the NDTF determined that a minimum sample size of 100 normal subjects was necessary for consideration of a particular published study and should be the first criterion for selecting articles from a literature search.
Subject Description. A high-quality normative data study on EDX parameters must be designed as a prospective study that controls sampling bias by establishing rigorous inclusion and exclusion criteria to ensure that asymptomatic healthy individuals are recruited while excluding those with confounding medical or surgical conditions known to affect the peripheral nervous system (e.g., diabetes, excessive alcohol use, previous carpal tunnel syndrome, previous median or ulnar surgery, to name a few).11–14 Use of a sample of persons referred to an EDX laboratory and found to have “normal” studies is not appropriate, because they were referred to the laboratory for a reason and are not truly “normal." 9,11 Studies in which the subjects are recruited from a specific population (such as factory workers) introduce strong sampling bias due to a predilection for people from certain demographics and occupations (such as susceptibility to entrapment neuropathies). In studies that used disease controls (e.g., studies on diabetic neuropathy) normative values obtained by testing non-diabetic individuals as healthy control subjects are acceptable if they meet the inclusion and exclusion thresholds.
The NDTF addressed only reference values in those >18 years old. Age is a known independent variable that affects NCS parameters, particularly in the elderly. Pediatric normative studies are different in nature and were not part of the scope of work of the NDTF.
Technical Factors. Technical factors impart the accurate measurement of NCS parameters for motor and especially sensory nerves, which have potentials 3 orders of magnitude smaller than those of motor nerves.14–16 Issues include proper electrode set-up, accurate distance measurements, and care to prevent excessive stimulation of adjacent nerves.16 Falck and Stalberg described the use of standardized NCS techniques and developed normative data at 3 Scandinavian institutions.15 They showed that reference values could be used across different laboratories when technical factors were “carefully standardized.”15
Temperature is recognized as an important factor in NCS testing, as reduced lower limb temperatures may cause slower motor and sensory conduction velocities, prolonged latencies, and higher amplitude responses.2,14–16 Despite this, methodologies for maintaining warm limbs are not well studied and are not standardized. The optimal location for monitoring temperature has not been studied to make firm recommendations. For instance, the temperature in the distal forearm is often different than the temperature at the digit. General recommendations include maintaining temperatures between 328 and 368C for upper limb testing and between 308 and 368C for the lower limb.2 Warm water baths, heating lamps, hydrocollator pads, and hair dryers can be used for maintaining limb temperature. Whatever technique is used, high-quality published studies should include specific temperature control measures.
Measurement of nerve length is prone to individual examiner error.17 Results from 100 EDX physicians who measured the average wrist-to thenar segment (median motor nerve) yielded a value of 6.3 6 0.7 cm (mean 6 SD), with a range of 5.0–10.0 cm; the wrist-to-index finger segment average was 13.9 6 0.3 cm, with a range of 13.0– 15.0 cm.17 Use of standardized fixed distances and specific electrode placement rather than anatomical landmarks is recommended by the NDTF. Normative articles must include clear descriptions of the stimulation and recording sites and the distances between these sites.12,13,18
Machine settings can contribute to variability in NCS testing.19 High- and low-frequency filter settings affect both onset latencies and amplitudes.12,13
Specification of these testing parameters is necessary to ensure high-quality studies.
A number of technical issues must be considered. Physical features of patients that influence nerve conduction recording include pedal edema and adipose tissue under the stimulating and recording electrodes. These factors cannot be entirely controlled. However, the skin should be clean and free of lotion. Preparing the skin with a mild abrasive paste should be considered to ensure excellent contact and low electrode impedance.
Supramaximal stimulation is essential but with care to avoid obscuring stimulus artifacts or stimulation of adjacent nerves.16 Because sensory nerve action potentials (SNAPs) are low amplitude, it is important to demonstrate a consistent and reproducible SNAP and to ensure that a true response is elicited and not a captured motor unit action potential, compound muscle action potential, or artifact. Background motor unit potential firing can lead to a captured response that looks similar to a SNAP.14,16 When SNAPs are low amplitude (e.g., <5 lV), signal averaging 4–6 responses can reduce the effects of background noise.
Subject Age and Physical Features. Age and physical features of the subject, such as body mass index and height, are variables that may influence nerve conduction parameters.7,11,16,20 The NDTF required that selected normative studies include samples with a wide distribution of subject ages and, in particular, that older age subsets were adequately represented. Further, the statistical methods and data representation must have clearly taken into consideration the influence of age in derivation of reference cut-off values.
Statistical and Methodological Issues. Statistical methods were addressed for normative studies with the aim of developing generalizable reference
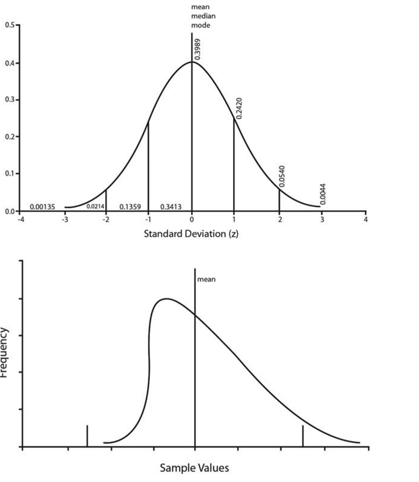
FIGURE 1. Frequency distribution curves for a hypothetical Gaussian variable (upper graph) and one with strong positive skew (lower graph). For the Gaussian distribution, the numbers to the right indicate representative frequency values, and those to the left indicate the area under the curve for each increment of the z-score (standard deviation). For the skewed distribution, the vertical bars indicate the values corresponding to 62.5 SD about the mean; because of the erroneous Gaussian assumption, too many cases fall above the upper limit, and the lower limit is not meaningful. (Reproduced with permission from the AANEM.11 values.7,9,11–13 Nerve conduction parameters often do not follow a Gaussian (normal) distribution but are skewed (Fig. 1),7,9,11,20 and Gaussian statistics cannot be used. Tests for normality and the use of alternative statistical methods that accommodate non-Gaussian data must be used.9,11 Methods to correct for non-normality include: (1) logarithmic or other appropriate mathematical transformation of the data for analysis; or (2) utilization of percentile cut-offs to define thresholds of abnormality.7,9,11,20 Normative studies should present their data such that the percentiles or cut-offs are clearly available. The percent of subjects who have an absent response should be reported but not used for cut-off calculations. Finally, reference values for NCS parameters must be clearly presented, preferably in a tabular format for ease of use by EDx physicians and technologists.
Another statistical consideration is overlapping distributions of data between normal samples and those with defined diseases or conditions.9,11 The NDTF did not define the overlap between diseased and normal populations but instead focused on studies that examined normal (asymptomatic) individuals and the statistical distributions of their nerve conduction parameters.
DISCUSSION
The NDTF has proposed a set of quality criteria (Table 1) to define the important methodological issues required of studies that investigate nerve conduction normative values. Although studies that meet these criteria may be few in number, these criteria can serve as benchmarks for future normative study designs. Editors and reviewers can use these criteria to evaluate such studies when considering them for publication.For common motor and sensory NCS the application of these criteria to the existing scientific literature and the final selected studies for recommendation are reported in a companion article.21
The NDTF members gratefully acknowledge the assistance of the AANEM Practice Issue Review Panel for review and critique of the manuscript. The NDTF also acknowledges Carrie Winter, Shirlyn Adkins, Seng Vang, Catherine French, and Adam Blaszkiewicz for their tireless administrative support of this project. The authors thank Larry Robinson, MD, and William Litchy, MD, for their thoughtful input in the early stages of this project. We also thank Katie McCausland, DO, for assistance with the ulnar and fibular (peroneal) motor nerves.
References
- American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine. Muscle Nerve 1999;22(suppl):S3–S300.
- Jablecki CK, Busis NA, Brandstater MA, Krivickas LS, Miller RG,Robinton JE. Reporting the results of needle EMG and nerve conduction studies: an educational report. Muscle Nerve 2005;32:682– 685.
- Delisa JA. Manual of nerve conduction velocity and clinical neurophysiology, 3rd ed. New York: Raven Press; 1994.
- Ma DM, Liveson JA. Nerve conduction handbook. Philadelphia: FADavis; 1983.
- Dyck P, Albers JW, Wolfe J, Bolton CF, Walsh N, Klein CJ, et al. A trial of proficiency of nerve conduction: greater standardization still needed. Muscle Nerve 2013;48:369–374.
- Litchy WJ, Albers JW, Wolfe J, Bolton CF, Walsh N, Klein CJ, et al. Proficiency of nerve conduction using standard methods and reference values (cl. NPhys Trial 4). Muscle Nerve 2014;50:900–908.
- Benatar M, Wuu J, Peng L. Reference data for commonly used sensory and motor nerve conduction studies. Muscle Nerve 2009;40:772–794.
- Chang AS, Dillingham TR, Yu KF. Statistical methods of computing reference values for side-to-side differences in nerve conduction studies. Am J Phys Med Rehabil 1996;75:437–442.
- Wang SH, Robinson L. Considerations in reference values for nerve conduction studies. PM&R Clin N Am 1998;9:907–923.
- Falck B, Andreassen S, Groth T, Lang H, Melander M, Nurmi A,et al. The development of a multicenter database for reference values in clinical neurophysiology—principles and examples. Comput Methods Progr Biomed 1991;34:145-162.
- Dorfman LJ, Robinson LR. AAEM Minimonograph #47: Normative data in electrodiagnostic medicine. Muscle Nerve 1997;20:4–14.
- Gitter AJ, Stolov WC. AAEM Minimonograph #16: Instrumentation and measurement in electrodiagnostic medicine, part I. Muscle Nerve 1995;18:799–811.
- Gitter AJ, Stolov WC. AAEM Minimonograph #16: Instrumentation and measurement in electrodiagnostic medicine, part II. Muscle Nerve 1995;18:812–824.
- Wilbourn A. Sensory conduction studies. J Clin Neurophysiol 1994;11:584.
- Falck B, Stålberg EV. Motor nerve conduction studies: measurement principles and interpretation of findings. J Clin Neurophysiol 1995;12:254.
- Kimura J. Principles and pitfalls of nerve conduction studies. AnnNeurol 1984;16:415.
- Roy PC, Wertsch JJ. Interexaminer variability of distance measurement in nerve conduction studies [abstract]. Muscle Nerve 2007;36:561.
- Maynard FM, Stolov WC. Experimental error in determination of nerve conduction velocity. Arch Phys Med Rehabil 1972;53:362–372.
- Nobushige T, Robinson L. Does display sensitivity influence motor latency determination? Muscle Nerve 2010;41;309–312.
- Peng L, Wuu J, Benatar M. Developing reference data for nerve conduction studies: an application of quantile regression. Muscle Nerve 2009;40:763–771.
- Chen S, Andary M, Buschbacher R, Del Toro D, Smith B, et al. Electrodiagnostic reference values for upper and lower limb nerve conduction studies in adult populations. Muscle Nerve (to appear).
Document History
Approved by the American Association of Neuromuscular & Electrodiagnostic Medicine: May 2016
This guideline is greater than 5 years old. Every 5 years, an interim literature search is performed and the guideline is reviewed. While new studies have been published since this guideline was last reviewed, the Practice Issue Review Panel Committee of the AANEM has determined that these studies are not sufficient to mandate a revision of this guideline at the present time. The information contained in this guideline and the recommendations offered are still relevant to current practice.
Reaffirmed by the Practice Issue Review Panel: October 2022Creation of New Guidelines, Consensus Statements, or Position Papers
AANEM members are encouraged to submit ideas for papers that can improve the understanding of the field. The AANEM will review nominated topics on the basis of the following criteria:- Members’ needs
- Prevalence of condition
- Health impact of condition for the individual and others
- Socioeconomic impact
- Extent of practice variation
- Quality of available evidence
- External constraints on practice
- Urgency for evaluation of new practice technology
