Consensus Statement: Using Laryngeal EMG for the Diagnosis and Treatment of Vocal Cord Paralysis
AANEM PRACTICE TOPIC
MICHAEL C. MUNIN, MD,1 YOLANDA D. HEMAN-ACKAH, MD, MS,2,3 CLARK A. ROSEN, MD,4 LUCIAN SULICA, MD,5 NICOLE MARONIAN, MD,6 STEVEN MANDEL, MD,7 BRIDGET T. CAREY, MD,8 EARL CRAIG, MD,9 and GARY GRONSETH, MD10
- Department of Physical Medicine and Rehabilitation, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA
- Department of Otolaryngology–Head and Neck Surgery, Drexel University College of Medicine, Philadelphia, Pennsylvania, USA
- Sidney Kimmel Medical College, Philadelphia, Pennsylvania, USA
- Department of Otolaryngology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA
- Department of Otolaryngology–Head & Neck Surgery, Weill Cornell Medical College, New York, New York, USA
- Ear, Nose and Throat Institute, Case Western Reserve University, Cleveland, Ohio, USA
- Department of Neurology, Hofstra North Shore LIJ School of Medicine, Hempstead, New York, USA
- Department of Neurology, Weill Cornell Medical College, New York, New York, USA
- Department of Physical Medicine and Rehabilitation, Indiana University School of Medicine, Indianapolis, Indiana, USA
- Department of Neurology, University of Kansas Medical Center, Kansas City, Kansas, USA
ABSTRACT
Introduction: The purpose of this study was to develop an evidence-based consensus statement regarding use of laryngeal electromyography (LEMG) for diagnosis and treatment of vocal fold paralysis after recurrent laryngeal neuropathy (RLN). Methods: Two questions regarding LEMG were analyzed: (1) Does LEMG predict recovery in patients with acute unilateral or bilateral vocal fold paralysis? (2) Do LEMG findings change clinical management in these individuals? A systematic review was performed using American Academy of Neurology criteria for rating of diagnostic accuracy. Results: Active voluntary motor unit potential recruitment and presence of polyphasic motor unit potentials within the first 6 months after lesion onset predicted recovery. Positive sharp waves and/or fibrillation potentials did not predict outcome. The presence of electrical synkinesis may decrease the likelihood of recovery, based on 1 published study. LEMG altered clinical management by changing the initial diagnosis from RLN in 48% of cases. Cricoarytenoid fixation and superior laryngeal neuropathy were the most common other diagnoses observed. Conclusions: If prognostic information is required in a patient with vocal fold paralysis that is more than 4 weeks and less than 6 months in duration, then LEMG should be performed. LEMG may be performed to clarify treatment decisions for vocal fold immobility that is presumed to be caused by RLN.
The mainstays in diagnosis of RLN are history and in-office flexible laryngoscopy, where clinical findings demonstrate an immobile vocal fold. Clinical evaluation alone cannot determine the prognosis for recovery or yield a definitive understanding of the underlying pathophysiology, which may, in turn, affect clinical decision-making. Laryngeal electromyography (LEMG), first described by Weddell in 1944, has been utilized increasingly to understand neuromuscular function after laryngeal nerve injury.10 Ideally, a team of an electrodiagnostic physician and an otolaryngologist perform LEMG as a brief, office-based procedure, to assist in decisionmaking about diagnosis, prognosis, and subsequent rehabilitative procedures. The diagnostic approach for needle electromyography of the intrinsic laryngeal adductor muscles involves transcutaneous insertion via the cricothyroid membrane. Information on laryngeal electrical activity is gathered at rest based on spontaneous activity followed by confirmatory phonatory tasks for motor unit potential (MUP) analysis.11,12 LEMG can identify changes in MUP recruitment and configuration that may change depending on the timing of the procedure relative to the date of the original lesion. Recovery from RLN is related to the severity of nerve injury depending on whether there is conduction block, axonal loss, or a combination of both.13 Understanding how information from LEMG corresponds to eventual recovery has been challenging to determine. This evidence-based review was designed to address 2 critical questions regarding the use of LEMG after RLN. First, does LEMG predict recovery in patients with acute unilateral or bilateral vocal fold paralysis? Second, do LEMG findings change clinical management or influence outcomes in these individuals?
DESCRIPTION OF THE ANALYTIC PROCEDURES
The American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) convened an expert panel of physicians who specialize in neurology, otolaryngology, and physical medicine and rehabilitation. This panel was selected to represent a broad range of expertise related to LEMG, and most participants reported using LEMG frequently for clinical and research purposes.
In October 2012, PubMed was used to search Medline to identify all potential abstracts. The search strategy for this study included the keywords, MeSH terms, and text words. The search terms included laryngeal electromyography, motor unit recruitment, fibrillation potentials, positive wave potentials, laryngeal synkinesis, turns-toamplitude ratio, quantitative electromyography, thyroarytenoid muscle, cricothyroid muscle, lateral cricoarytenoid muscle, posterior cricoarytenoid muscle, recurrent laryngeal nerve injury, recurrent laryngeal neuropathy, and vocal fold paralysis. This produced 1,540 English-only abstracts that matched the search terms, including human and highly relevant animal studies and all age groups, published between 1960 and October 2012. Use of LEMG for intraoperative monitoring of nerve activity was an exclusion criterion. Titles were reviewed for relevance, which yielded 273 articles. At least 2 investigators then reviewed abstracts for 254 publications, because 19 could not be located. This level of review resulted in 65 publications for full manuscript data abstraction. After each was reviewed in its entirety by 2 investigators, 14 were identified as relevant for this guideline. To be considered relevant, the article had to describe both: (1) patients with a neurologic disease affecting the laryngeal muscles; and (2) subjects with and without LEMG abnormalities. The results of the LEMG (the index test) had to be compared with the reference standard of recovery of vocal fold motion as detected by laryngoscopy.
All included publications evaluated a minimum of 10 subjects and described the LEMG technique in detail. Studies on clinical management were required to describe how patient treatment was altered by the results of the LEMG. Studies that evaluated whether LEMG predicted recovery of vocal fold mobility were required to use laryngoscopy at the onset of symptoms and at interval recovery periods until at least 6 months after onset of symptoms. If the initial LEMG was performed >6 months after onset of injury, the data for those individual patients were excluded, because the correlation of late LEMG studies to outcomes is known to be low.12 Late LEMG prognostic information does not add further value, as spontaneous recovery after 6 months of paralysis is quite rare. In addition, synkinetic reinnervation could yield normal MUP recruitment without any vocal fold motion.
The 14 relevant publications were rated using the American Academy of Neurology grading system. At each step in the process, disagreements were arbitrated by a third investigator. Some articles focused strictly on unilateral vocal fold paralysis, and some included both unilateral and bilateral paralysis. When information regarding bilateral vocal fold paralysis was reported, each individual nerve served as a separate data point in the analysis.
ANALYSIS OF THE EVIDENCE
LEMG to Predict Recovery after RLN. Meta-analysis was performed for 4 variables using a random effects model to calculate 95% confidence intervals of the risk differential. The 4 variables were positive predictive value, negative predictive value, sensitivity, and specificity, which were calculated from the 3 parameters that were most commonly investigated as predictors of recovery: presence of MUPs; absence of spontaneous activity (specifically absence of positive sharp waves and/or fibrillation potentials); and presence of polyphasic MUPs. Of note, an abnormal percentage of polyphasic MUPs was described clinically in each study, but there is no uniform definition for this parameter. One study investigated the absence of electrical synkinesis as a predictor of recovery.14
One study was a prospective, randomized, controlled trial in animals in which vocal fold paralysis was surgically induced, and LEMG and laryngoscopy were performed at the onset of injury and in follow-up.15 Because there was only 1 animal study identified as pertinent to the research question, the data from this study were not used to calculate the 95% confidence intervals in an effort to minimize bias in the random effects model.15
Three studies were excluded from meta-analysis because of spectrum bias.14,16,17 Sittel et al. classified individuals who had mild paresis as being “not recovered,” whereas the other studies considered this function as a sign of recovery.16 Smith et al. excluded patients who had a poor prognosis, and data regarding whether or not any of these individuals recovered are not available.17 Statham et al. excluded patients with severely decreased or absent recruitment and did not provide any data on whether or not any of these individuals had recovery of function.14 In addition, the Stager and Bielamowicz study was excluded from analysis, because it did not separate patients who had vocal fold paralysis from those with paresis.18 The Grosheva et al. study was excluded from analysis because the outcome of interest was electrical activity and not vocal fold mobility.19
Table 1. Predictive value, sensitivity, and specificity for presence of motor unit potentials in predicting recovery.
| Publication | Number of nerves | Positive predictive value | Negative predictive value | Sensitivity | Specificity |
| Parnes and Satyamurti23 | 26 | 0.55 | 1.00 | 1.00 | 0.40 |
| Gupta and Bastian21 | 17 | 0.67 | 0.63 | 0.67 | 0.63 |
| Min et al.22 | 9 | 0.50 | 0.80 | 0.67 | 0.67 |
| Munin et al.24 | 31 | 0.55 | 0.85 | 0.67 | 0.77 |
| Hydman et al.26 | 15 | ND | ND | ND | ND |
| Wang et al.25 | 45 | 0.63 | 0.78 | 0.38 | 0.91 |
| Elez and Celik27 | 20 | 0.73 | 1.00 | 1.00 | 0.56 |
ND, no data were presented for this variable in this study.
A total of 9 articles addressed the issue of whether or not LEMG helps to predict recovery of vocal fold motion. In a controlled study, LEMG results of 35 patients with unilateral immobile vocal folds were compared with those of 10 control sub- jects.20 Concentric electrodes were used for this study. Follow-up LEMG and flexible fiber-optic lar- yngoscopy were performed at 6 months. In this study, the absence of fibrillation potentials and posi-tive sharp waves in the first 6 months after injury had a positive predictive value for recovery of 63% and a sensitivity of 100%. The negative predictive value (i.e., the presence of positive sharp waves and/or fibrillation potentials as a predictor of no recovery of vocal fold mobility) was 100%, and the specificity was 60%. In addition, the presence of polyphasic MUPs in the first 6 months of recovery had a positive predictive value for recovery of 80% and a sensitivity of 80%. The negative predictive value of absence of polyphasic motor unit potentials as a predictor of failure to recover vocal fold mobility was 87%, with specificity also 87%.
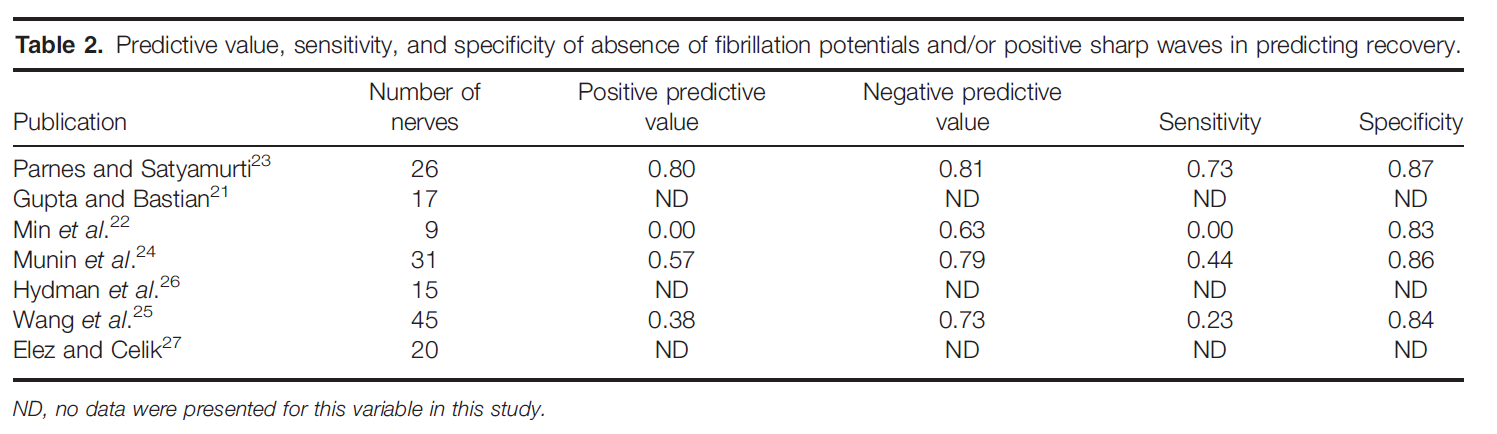
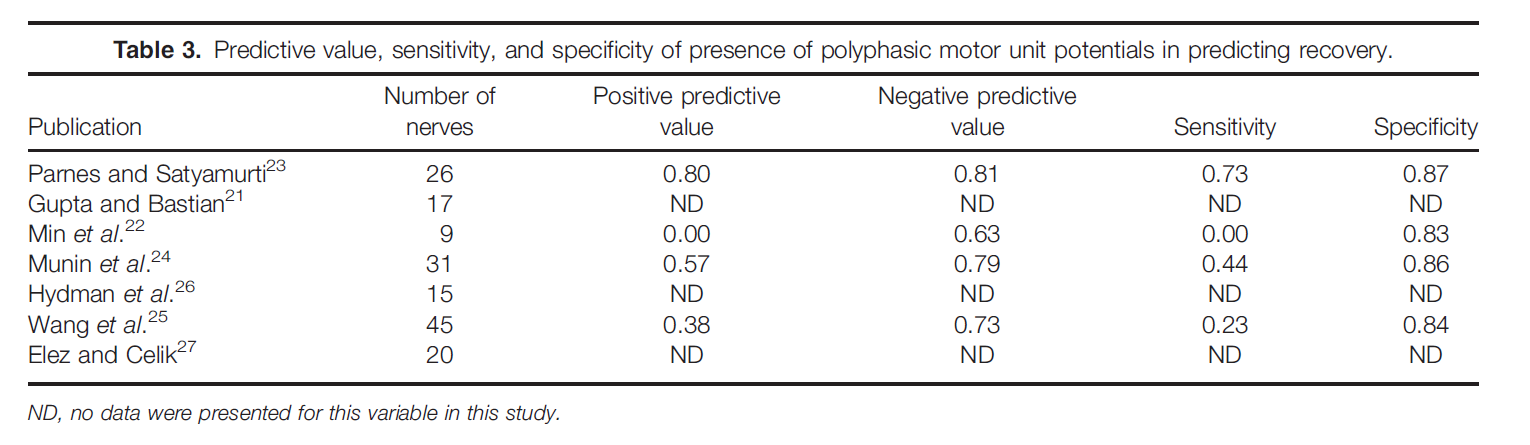
In the study by Gupta and Bastian, 1 patient was excluded because LEMG was performed at 8 months postinjury.21 There was no recovery in this individual and no fibrillation potentials, but voluntary MUPs were present. In the study by Min etal., 5 patients were excluded because their follow-up LEMG was performed at >6 months postinjury.22 Bipolar electrodes were used in 3 studies,15,22,23 monopolar electrodes were used in 2 studies,24,25 concentric electrodes were used in 1 study,26 and electrode type was not mentioned in 2 studies.21,27 Table 1 shows the data from each individual study regarding the presence of MUPs as a predictor for recovery. Meta-analysis of these data indicates that the presence of MUPs increased the likelihood of recovery by 52.6% over their absence (absolute risk decrease 5 0.526, 95% confidence interval 0.387–0.664, P < 0.05; Fig. 1). Table 2 shows the data from each individual study regarding use of absence of fibrillation potentials and/or positive sharp waves for predicting recovery. Meta-analysis of these data indicates that there is insufficient evidence to determine the usefulness of the presence of fibrillation potentials and/or positive sharp waves for predicting recovery of vocal fold mobility (absolute risk increase 5 0.234, 95% confidence interval 0.002–0.467, P < 0.05). Table3 shows data from each individual study regarding presence of polyphasic MUPs as a predictor of recovery. Meta-analysis of these data indi- cates that the presence of polyphasic MUPs increases the likelihood of recovery by 44.8% over their absence in the first 6 months after injury (absolute risk decrease 5 0.448, 95% confieinterval 0.217–0.681, P < 0.05).
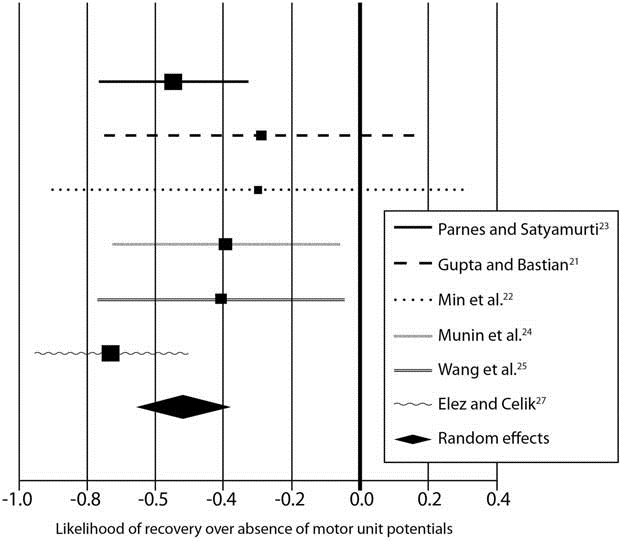
FIGURE 1. Meta-analysis indicates that the presence of motor unit potentials on laryngeal electromyography increases the likeli- hood of recovery. The graph shows individual studies compared with the random effects (as indicated by diamond symbol).
Statham et al. showed that the absence of electrical synkinesis was a predictor of recovery in patients with vocal fold immobility who demonstrated normal or nearnormal MUP recruitment during vocalization.14 The absence of electrical synkinesis, which was defined as MUP amplitude during a sniff (abduction task) / MUP amplitude during phonation (adduction task) <0.65 when recording signals in the thyroarytenoid muscle, had a positive predictive value for recovery of 68% and a sensitivity of 93%. The presence of electrical synkinesis had a negative predictive value for recovery of 92% and a specificity of 67%. The absolute risk increase for the presence of electrical synkinesis was 60.7% (absolute risk increase = 0.607, 95% confidence interval 0.352–0.861, P < 0.05), indicating that the presence of synkinesis increased the likelihood of vocal fold immobility over its absence by 60.7%.
Conclusion. LEMG can predict outcome after RLN. The individual parameters of the LEMG study that determine return of vocal fold motion include active voluntary MUP recruitment and the presence of polyphasic MUPs within the first 6 months after injury. Positive sharp waves and/or fibrillation potentials did not predict outcome. The presence of electrical synkinesis may decrease the likelihood of recovery, based on 1 published study.
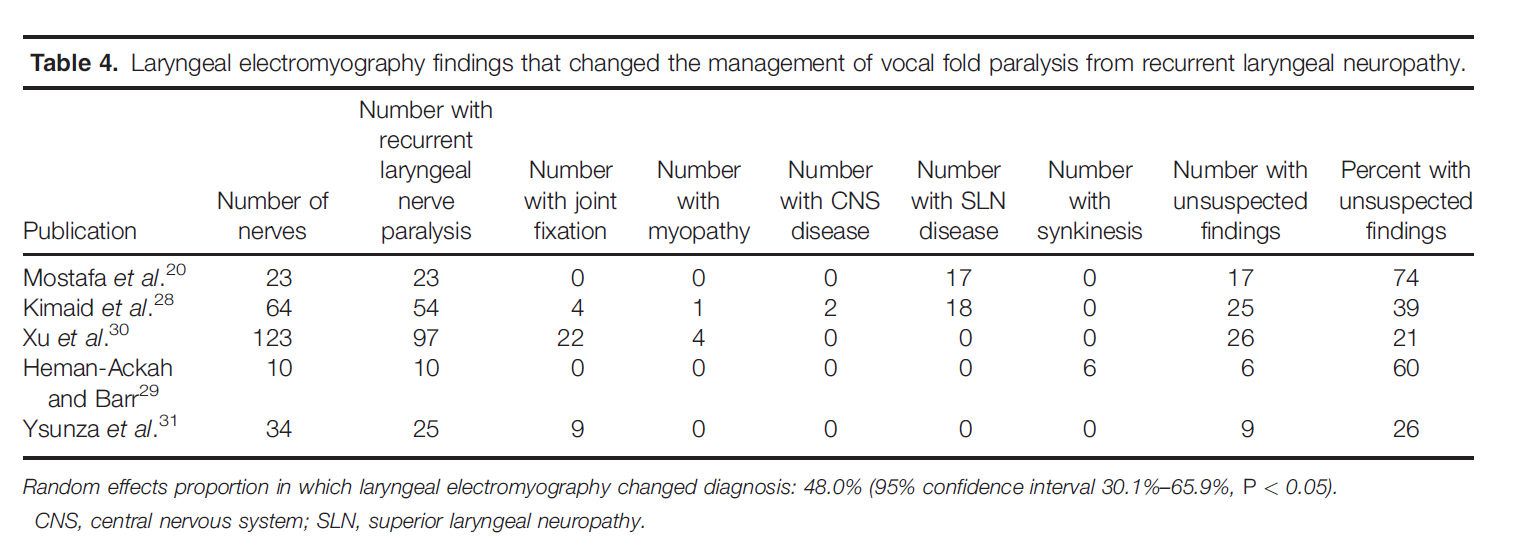
LEMG Findings that Changed Clinical Management. Five studies had data that addressed whether or not LEMG changed clinical management.20,28–31 Diagnostic accuracy is not addressed here, because there is no independent reference test for this condition. In these studies, the commonly held pre-test assumption was that vocal fold immobility was due to RLN dysfunction. Clinical management was subsequently changed if this diagnosis was not confirmed by LEMG testing. A random effects meta-analysis showed that, when LEMG was performed, clinical management was changed in 48% of cases (95% confidence interval 30.1%–65.9%). In other words, a clinical care plan was altered 48% of the time because the LEMG switched the diagnosis from RLN to another diagnosis or to an additional diagnosis. The data for these individual studies are presented in Table 4. Clinical management was changed most often when the LEMG results implied superior laryngeal neuropathy, cricoarytenoid joint fixation, myopathy, and stroke. The latter condition was suggested based on normal MUP configuration in superior and recurrent laryngeal-innervated muscles but decreased recruitment to task without increased firing rates.
Conclusion. LEMG adds value by changing the clinical management of a patient with vocal fold paralysis approximately 48% of the time by suggesting diagnoses other than RLN. Cricoarytenoid fixation and superior laryngeal neuropathy were the most common diagnoses observed when the pre- test impression was RLN.
CLINICAL CONTEXT
LEMG ideally is performed by an otolaryngologist in combination with a board-certified electrodiagnostic physician, and both members of the LEMG team should be blinded to the laterality of the suspected lesion to prevent bias. LEMG conclusions are based on prognostic information obtained between 4 weeks and 6 months from onset of vocal fold paralysis.
There are less common clinical situations in which the differential diagnosis for an immobile vocal fold includes an RLN versus cricoarytenoid joint dislocation or fixation. This can occur in cases of vocal fold immobility after recent endotracheal intubation with no clear etiology for an iatrogenic nerve injury to the vagus and/or recurrent laryngeal nerves. LEMG can provide clarity between the clinical presentation of RLN versus mechanical cricoary- tenoid joint abnormality, because the latter would have normal LEMG findings. If the patient with uni- lateral vocal fold paralysis has only minimal functional impairment of voice, breathing, or swallowing function, LEMG generally is not necessary. Treatment of unilateral vocal fold paralysis consists of procedures to reposition the paralyzed vocal fold to improve or restore glottic competence, thereby improving the symptoms of this disorder. Different surgical procedures may provide either temporary or permanent relief of symptoms from unilateral vocal fold paralysis.
Prognostic information is of obvious importance in deciding whether to observe or implement corrective surgical procedures. If LEMG data show signs of reinnervation and recovery, then this can inform the patient and clinician to pursue a continued period of observation or to use a temporary treatment (e.g., vocal fold injection with a material that dissipates in 2–3 months). If the LEMG data reveal a poor prognosis based on lesion severity, permanent surgical treatment can be offered sooner for appropriate patients. Treatment of bilateral vocal fold paralysis generally is irreversible, involving destruction of some part of the vocal fold and/or arytenoid to enlarge the glottic airway. Information provided by LEMG before embarking on this permanent surgical treatment also may help select a side for the surgical intervention.
SUMMARY RECOMMENDATIONS
- If prognostic information is required on ultimate vocal fold mobility in a patient with vocal fold paralysis that is >4 weeks and <6 months in duration, LEMG should be performed.
- LEMG may be performed to clarify treatment decisions in a patient with vocal fold immobility that is presumed to be caused by RLN.
RECOMMENDATIONS FOR FUTURE RESEARCH
Refinement of the LEMG technique would help improve diagnostic accuracy. For example, in a patient with anterior neck surgery and local swelling, it can be challenging to find the cricothyroid membrane needle electrode placement into the thyroarytenoid/lateral cricoarytenoid muscle complex. Image guidance using diagnostic ultrasound should be evaluated to see if it can improve needle localization. Recurrent laryngeal nerve motor conduction studies should be developed to complement LEMG testing. Current techniques are hampered by volume conduction from stimulation in the neck and the inability to record compound muscle action potentials in the intrinsic laryngeal muscles transcutaneously through the thyroid cartilage.
The natural history and mechanisms of laryngeal synkinesis are not well understood. Further research to understand, diagnose, and ultimately minimize this process through rehabilitative training or medications would be an advancement in the field. Last, future prospective studies should examine patient outcomes regarding clinical decisions that were made using LEMG data as compared with decisions made without this information. To avoid potential bias, LEMG testing should employ blinding regarding laterality of the immobile vocal fold and blinding when performing follow-up visual laryngoscopic assessment of vocal fold motion.
References
- Weisberg NK, Spengler DM, Netterville JL. Stretch induced nerve injury as a cause of paralysis secondary to the anterior cervical approach. Otolaryngol Head Neck Surg 1997;116:317–326.
- Netterville JL, Koriwchak MJ, Winkle M, Courey MS, Ossoff RH. Vocal fold paralysis following anterior approach to the cervical spine. Ann Otol Rhinol Laryngol 1996;105:85–91.
- Sugarbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, et al. Prevention, early detection and management of complication after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138–146.
- Filiare M, Mom T, Laurent S, Harouna Y, Naamee A, Vallet L, et al. Vocal cord dysfunction after left lung resection for cancer. Eur J Car- diothorac Surg 2001;20:705–711.
- Dimarakis I, Protopapas AD. Vocal cord palsy as a complication of adult cardiac surgery: surgical correlations and analysis. Eur J Cardio- thorac Surg 2004;26:773–775.
- Gockel I, Kneist W, Keilman A, Junginger T. Recurrent laryngeal nerve paralysis (RLNP) following esophagectomy for carcinoma. Eur J Surg Oncol 2005;31:277–281.
- Netterville JL, Jackson CJ, Miller FR, Wanamaker JR, Glasscock ME. Vagal paraganglioma: a review of 46 patients treated during a 20 year period. Arch Otolaryngol Head Neck Surg 1998;124:1133–1140.
- Zambudio AR, Rodriguez J, Riquelme J, Soria T, Canteras M, Parrilla P. Prospective study of postoperative complications after total thy- roidectomy for multinodular goiters by surgeons with experience in endocrine surgery. Ann Surg 2004;240:18–25.
- Steurer M, Passler C, Denk DM, Schneider B, Niederele B, Bigenzahn W. Advantages of recurrent laryngeal nerve identification in thyroidectomy and parathyroidectomy and the importance of pre- operative and postoperative laryngoscopic examination in more than 1000 nerves at risk. Laryngoscope 2002;112:124–133.
- Weddell G, Feinstein B, Pattle RE. The electrical activity of voluntary muscle in man under normal and pathological conditions. Brain 1944;67:178–257.
- Buchtal F. Electromyography of intrinsic laryngeal muscles. Q J Exp Physiol Cogn Med Sci 1959;44:137–148.
- Hiroto I, Hirano M, Tomita H. Electomyographic investigations of human vocal cord paralysis. Ann Otol 1968;77:296–304.
- Blitzer A, Jahn AF, Keidar A. Semon’s law revisited: an electromyo- graphic analysis of laryngeal synkinesis. Ann Otol Rhinol Laryngol 1996;105:764–769.
- Statham MM, Rosen CA, Smith LJ, Munin MC. Electromyographic laryngeal synkinesis alters prognosis in vocal fold paralysis. Laryngo- scope 2010;120:285–290.
- Mu L, Yang S. An experimental study on the laryngeal electromyog- raphy and visual observations in varying types of surgical injuries to the unilateral recurrent laryngeal nerve in the neck. Laryngoscope 1991;101:699–708.
- Sittel C, Stennert E, Thumfart WF, Dapunt U, Eckel HE. Prognostic value of laryngeal electromyography in vocal fold paralysis. Arch Oto- laryngol Head Neck Surg 2001;127:155–160.
- Smith LJ, Rosen CA, Niyonkuru C, Munin MC. Quantitative electro- myography improves prediction in vocal fold paralysis. Laryngoscope 2012;122:854–859.
- Stager SV, Bielamowicz SA. Evidence of return of function in patients with vocal fold paresis. J Voice 2010;24:614–622.
- Grosheva M, Wittekindt C, Potoschnig C, Lindenthaler W, Guntinas- Lichius O. Evaluation of peripheral vocal cord paralysis by electromy- ography. Laryngoscope 2008;118:987–990.
- Mostafa BE, Gadallah NA, Nassar NM, Ibiary HMA, Fahmy HA, Fouda NM. The role of laryngeal electromyography in vocal fold immobility. ORL J Otorhinolaryngol Relat Spec 2004;66:5–10.
- Gupta SR, Bastian RW. Use of laryngeal electromyography in prediction of recovery after vocal cord paralysis. Muscle Nerve 1993;16:977–978.
- Min YB, Finnegan EM, Hoffman HT, Luschei ES, McCulloch TM. A preliminary study of the prognostic role of electromyography in laryngeal paralysis. Otolaryngol Head Neck Surg 1994;111:770–775.
- Parnes SM, Satyamurti SM. Predictive value of laryngeal electromyog- raphy in patients with vocal cord paralysis of neurogenic origin. Laryngoscope 1985;95:1323–1326.
- Munin MC, Rosen CA, Zullo T. Utility of laryngeal electromyography in predicting recovery after vocal fold paralysis. Arch Phys Med Reha- bil 2003;84:1150–1153.
- Wang CC, Chang MH, Wang CP, Liu SA. Prognostic indicators of unilateral vocal fold paralysis. Arch Otolaryngol Head Neck Surg 2008;134:380–388.
- Hydman J, Bjorck G, Persson JKE, Zedenius J, Mattsson P. Diagnosis and prognosis of iatrogenic injury of the recurrent laryngeal nerve. Ann Otol Rhinol Laryngol 2009;118:506–511.
- Elez F, Celik M. The value of laryngeal electromyography in vocal cord paralysis. Muscle Nerve 1998;21:552–553.
- Kimaid PA, Crespo AN, Quagliato EMAB, Wolf A, Viana MA, Resende LAL. Laryngeal electromyography: contribution to vocal fold immobility diagnosis. Electromyogr Clin Neurophysiol 2004;44: 371–374.
- Heman-Ackah YD, Barr A. The value of laryngeal electromyography in the evaluation of laryngeal motion abnormalities. J Voice 2006;20: 452–460.
- Xu W, Han D, Hou L, Zhang L, Zhao G. Value of laryngeal electro- myography in diagnosis of vocal fold immobility. Ann Otol Rhinol Laryngol 2007;116:576–581.
- Ysunza A, Landeros L, Pamplona C, Prado H, Arrieta J, Fajardo G. The role of laryngeal electromyography in the diagnosis of vocal fold immo- bility in children. Int J Pediatr Otorhinolaryngol 2007;71:949–958.
Document History
Approved by the American Association of Neuromuscular & Electrodiagnostic Medicine: February 2016.
This guideline is greater than 5 years old. Every 5 years, an interim literature search is performed and the guideline is reviewed. While new studies have been published since this guideline was last reviewed, the Practice Issue Review Panel Committee of the AANEM has determined that these studies are not sufficient to mandate a revision of this guideline at the present time. The information contained in this guideline and the recommendations offered are still relevant to current practice.
Reaffirmed by the Practice Issue Review Panel: October 2022.
Muscle Nerve 53: 850–855, 2016
Creation of New Guidelines, Consensus Statements, or Position Papers
AANEM members are encouraged to submit ideas for papers that can improve the understanding of the field. The AANEM will review nominated topics on the basis of the following criteria:- Members’ needs
- Prevalence of condition
- Health impact of condition for the individual and others
- Socioeconomic impact
- Extent of practice variation
- Quality of available evidence
- External constraints on practice
- Urgency for evaluation of new practice technology
