Treatment of Painful Diabetic Neuropathy
AANEM PRACTICE TOPIC
EVIDENCE-BASED GUIDELINE: TREATMENT OF PAINFUL DIABETIC NEUROPATHY—REPORT OF THE AMERICAN ASSOCIATION OF NEUROMUSCULAR AND ELECTRODIAGNOSTIC MEDICINE, THE AMERICAN ACADEMY OF NEUROLOGY, AND THE AMERICAN ACADEMY OF PHYSICAL MEDICINE & REHABILITATION
University Health Network, University of Toronto, Toronto, Ontario, Canada
Department of Neurology, Louisiana State University School of Medicine, New Orleans, Louisiana, USA
University of Washington, Seattle, Washington, USA
University of Wisconsin, Madison, Wisconsin, USA
Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire, USA
Department of Physical Medicine and Rehabilitation, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
University of Michigan, Ann Arbor, Michigan, USA
Humboldt Neurological Medical Group, Inc., Eureka, California, USA
Department of Neurology, University of Maryland School of Medicine, Baltimore, Maryland, USA
University of Calgary, Calgary, Alberta, Canada
ABSTRACT: The objective of this report was to develop a scientifically sound and clinically relevant evidence-based guideline for the treatment of painful diabetic neuropathy (PDN). The basic question that was asked was: What is the efficacy of a given treatment (pharmacological: anticonvulsants, antidepressants, opioids, others; non-pharmacological: electrical stimulation, magnetic field treatment, low-intensity laser treatment, Reiki massage, others) to reduce pain and improve physical function and quality of life (QOL) in patients with PDN? A systematic review of literature from 1960 to August 2008 was performed, and studies were classified according to the American Academy of Neurology classification of evidence scheme for a therapeutic article. Recommendations were linked to the strength of the evidence. The results indicate that pregabalin is established as effective and should be offered for relief of PDN (Level A). Venlafaxine, duloxetine, amitriptyline, gabapentin, valproate, opioids (morphine sulfate, tramadol, and oxycodone controlled-release), and capsaicin are probably effective and should be considered for treatment of PDN (Level B). Other treatments have less robust evidence, or the evidence is negative. Effective treatments for PDN are available, but many have side effects that limit their usefulness. Few studies have sufficient information on their effects on function and QOL.
Muscle Nerve 000: 000–000, 2011
Diabetic sensorimotor polyneuropathy represents a diffuse, symmetrical, and length-dependent injury to peripheral nerves that has major implications with regard to quality of life (QOL), morbidity, and costs from a public health perspective.1,2 Painful diabetic neuropathy (PDN) affects 16% of patients with diabetes, and it is frequently unreported (12.5%) and more frequently untreated (39%).3 PDN presents an ongoing management problem for patients, caregivers, and physicians. There are many treatment options available, and a rational therapeutic approach to the patient with PDN requires an understanding of the evidence for each intervention.
This article is a joint report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. It was approved by the AANEM Board of Directors on February 15, 2011. This report did not undergo further editorial review by Muscle & Nerve.
Abbreviations: EQ-5D, Euro-QOL (5 dimensions); NNT, number needed to treat; PDN, painful diabetic neuropathy; QOL, quality of life; RCT, randomized, controlled trial; SF-MPQ, Short Form of the McGill Pain Questionnaire; SF-QOL, Short Form of the Quality of Life Assessment; TENS, transcutaneous electrical nerve stimulation; VAS, visual-analog pain scale
Key words: efficacy; neuropathic pain; painful diabetic neuropathy; quality of life; treatment options
Disclaimer: This report is provided as an education service of the AANEM, the AAN, and the AAMP&R. It is based on an assessment of current scientific and clinical information. It is not intended to include all possible proper methods of care for a particular neurological problem or all legitimate criteria for choosing to use a specific procedure. Neither is it intended to exclude any reasonable alternative methodologies. The AANEM, AAN, and AAPM&R recognize that specific care decisions are the prerogative of the patient and physician caring for the patient, based on all of the circumstances involved.
Disclosures: V.B. has received research support from Talecris, Eisai, and Johnson & Johnson. J.D.E. has received honoraria from Talecris for consulting work and speaking; holds financial interests in Pfizer; and has received research support from Wyeth, the NIH, Astra Zeneca, and Pfizer. M.B. is on the editorial boards of Pain, Journal of Pain, Clinical Journal of Pain, European Journal of Pain, and Pain Medicine; he provided consultation to and holds corporate appointments with Allergan, Astellas, J&J, Lilly, Merck, Neurogesx, and Pfizer, and conducted clinical research with Neurogesx. J.A.C. has received funding for travel from the National ALS Association; has received honoraria from a neurology practice in Massachusetts; serves on the speakers’ bureau of Athena Diagnostics; and has given expert testimony, prepared an affidavit, and acted as a witness in a legal proceeding with regard to vaccine-related injuries and peripheral nerve injuries. E.L.F. has served as a journal editor for the Journal of the Peripheral Nervous System, Endocrinology, and Neurobiology of Disease; has received royalties from UpToDate; has received honoraria from the Neurological Institute of New York, the Detroit Medical Center, and the University of Pennsylvania; is on the Data Safety Monitoring Board of Novartis; and receives research support from the NIH. D.J.I. has been a treating expert witness with regard to a legal proceeding. J.W.R. has received financial compensation from Exelexis Corp. and Baxter Corp.; has received honoraria from the AAN and several hospitals and academic institutions; and has received research support from Baxter Corporation, the NIH, the VA, the American Diabetes Association, and the Juvenile Diabetes Association. D.W.Z. serves on the advisory board for Aegera, Inc.; has received honoraria from ONO, Japan; and holds stock options in and receives research support from Aegera.
DESCRIPTION OF THE ANALYTIC PROCESS
In January 2007, the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM), the American Academy of Neurology (AAN), and the American Academy of Physical Medicine and Rehabilitation (AAPM&R) convened an expert panel from the USA and Canada, selected to represent a broad range of relevant expertise. In August 2008, a literature search of MEDLINE and EMBASE was performed in all languages using the MeSH term diabetic neuropathies and its text word synonyms and key words for the therapeutic interventions of interest (see Appendix E1 for a full list of search terms). The search identified 2234 citations, the titles and abstracts of which were reviewed by at least two investigators for relevance, resulting in 463 articles. All of these articles were reviewed in their entirety and, of these, the panel identified 79 relevant articles. Each of these articles was rated by at least two investigators according to the AAN criteria for the classification of therapeutic articles (Appendix E2), and recommendations were linked to the strength of evidence (Appendix E3) and to effect size of the intervention. Disagreements regarding classification were arbitrated by a third reviewer.
Articles were included if they dealt with the treatment of PDN, described the intervention clearly, reported the completion rate of the study, and defined the outcome measures clearly. The panel also considered the side effects of the treatment and measures of function and QOL, if any. Case reports and review studies were excluded.
We anticipated that studies would use varying measures for quantifying pain reduction. For the purposes of this guideline we preferred the following outcome measures, listed in order of preference:
| 1. | The difference in the proportion of patients reporting a >30–50% change from baseline on a Likert or visual-analog pain scale (VAS) as compared with no treatment (placebo) or the comparative treatment. The Likert scale is an 11-point linear scale ranging from 0 (no pain) to 10 (maximum pain), and the patient rates his/her pain level on this scale.4–6 |
| 2. | The percent change from baseline on a Likertor VAS as compared with no treatment (placebo) or the comparative treatment.6 |
| 3. | Any other quantitative measure of pain reduction provided by the investigators. |
For studies reporting the difference in the proportion of patients reporting a >30–50% reduction in pain, we considered a risk difference of >20% to be a large effect [number needed to treat (NNT) <5], a risk difference of >10–20% (NNT >5–10) a moderate effect, and a risk difference of 10% (NNT >10) a small effect, where risk difference is the reduction in pain in the active treatment group minus the reduction in the control group. For studies using a mean reduction from baseline on a Likert scale or VAS as compared with no treatment (placebo) or a comparative treatment, we considered a reduction difference of >30% a large effect, >15% to 30% a moderate effect, and 15% a small effect. For any other quantitative measure of pain reduction, we considered a reduction of >30% a large effect, >15% to 30% a moderate effect, and 15% a small effect.
The panel recognized that older studies generally lacked measures of QOL and function compared with more recent studies. Furthermore, the panel was aware that a standardized QOL measure for PDN or a standardized assessment of function are not available, and multiple instruments were used to measure QOL, such as the 36-item Short Form (SF-36) Health Survey, subsections of the SF36, and function (such as sleep interference).
Studies with the highest levels of evidence for each intervention are discussed in the text, and others are shown in the tables. Details of Class I, II, and III studies are presented in the evidence tables.
ANALYSIS OF EVIDENCE
In patients with PDN, what is the efficacy of pharmacological agents to reduce pain and improve physical function and QOL?
Anticonvulsants. We identified 20 articles relevant to anticonvulsants graded higher than Class IV (Supplementary table 1). Most of the randomized, controlled trials (RCTs) rated as Class II instead of Class I had completion rates of < 80%, or the completion rate was not identified.
Four studies (three Class I and one Class II) evaluated the efficacy of pregabalin.7–10 All of the studies found that pregabalin relieved pain, but the effect size was small relative to placebo, reducing pain by 11–13% on the 11-point Likert scale in the Class I studies. A large dose-dependent effect (24–50% reduction in Likert pain scores compared with placebo) was observed in the Class II study.10 The NNT for a 50% reduction in pain was 4 at 600 g/day.7–10 In the QOL measures, social functioning, mental health, bodily pain, and vitality improved, and sleep interference decreased, all changes with P< 0.05.
Two studies (1 Class I and 1 Class II) evaluated the efficacy of gabapentin.11,12 In the Class I study,11 gabapentin had a small effect of net pain reduction from baseline of 11% on the 11-point Likert scale compared with the change in placebo treated patients, whereas a Class II gabapentin study showed no effect.12 Gabapentin had no effect on overall QOL in the single study reporting this measure, but it did show an improvement in subsets of mental health and vitality.11
Two Class I trials evaluated the efficacy of lamotrigine.13,14 There was no difference in the primary outcome measures in the lamotrigine and placebo groups.
Two studies (both Class II) evaluated the efficacy of sodium valproate.15,16 Both showed a 27– 30% pain reduction (moderate) in the Short Form McGill Pain Questionnaire (SF-MPQ) with sodium valproate compared with placebo, and QOL was not measured. Both studies were conducted at the same center with the same principal investigator in separate populations with small numbers of patients in each study and were remarkable for the lack of any change in placebo patients and for the lack of side effects typically attributed to sodium valproate. Treatment allocation concealment was not described.
One Class II study evaluated the efficacy of topiramate.17 The study reported a small effect compared with placebo, 7% net pain reduction on the VAS, and an NNT of 6.6 for >30% pain reduction.
Three Class II studies evaluated the efficacy of oxcarbazepine.18–20 Two studies showed no benefit,18,20 but a third showed a moderate benefit— 17% more patients on oxcarbazepine had a >50% pain reduction compared with placebo, with an NNT of 6.023.19 The study showing a positive response had a slightly higher completion rate (73%19 compared with 67%).20 Short Form Quality of Life (SF-QOL) was not improved.
Three Class III studies evaluated the efficacy of lacosamide.21–23 All the studies showed a small reduction in pain with 400 mg/day lacosamide (3%, 6%, and 6% compared with placebo), but in two studies no significant differences compared with placebo were observed with 600 mg/day lacosamide.22,23 In one study, benefits on general activity and sleep interference QOL measures were observed.21
Conclusions. Based on consistent Class I evidence, pregabalin is established as effective in lessening the pain of PDN. Pregabalin also improves QOL and lessens sleep interference, although the effect size is small. Based on one Class I study, gabapentin is probably effective in lessening the pain of PDN. Based on two Class II studies, sodium valproate is probably effective in treating PDN. Lamotrigine is probably not effective in treating PDN. Based on Class II evidence, oxcarbazepine is probably not effective in treating PDN. There is conflicting Class III evidence for the effectiveness of topiramate in treating PDN. Based on Class III evidence, lacosamide is possibly not effective in treating PDN. The degree of pain relief afforded by anticonvulsant agents is not associated with improved physical function. Recommendations:
| 1. | If clinically appropriate, pregabalin should be offered for the treatment of PDN (Level A). |
| 2. | Gabapentin and sodium valproate should be considered for the treatment of PDN (Level B). |
| 3. | There is insufficient evidence to support or refute the use of topiramate for the treatment of PDN (Level U). |
| 4. | Oxcarbazepine, lamotrigine, and lacosamide should probably not be considered for the treatment of PDN (Level B). |
Antidepressants. We identified 14 articles relevant to antidepressants rated higher than Class IV (Supplementary table 2). Seventeen articles were excluded. Most of the RCTs rated as Class II instead of Class I had completion rates of <80%.
Two studies (one Class I and one Class II) evaluated the efficacy of venlafaxine.24,25 The Class I study reported a moderate effect of venlafaxine, with 23% more pain relief than placebo on the VAS-PI (0–100) scale and an NNT of 5.24 In the Class II study, venlafaxine plus gabapentin showed a moderate effect in relieving pain on the 11-point Likert scale in PDN, with 18% more relief than placebo plus gabapentin.25 The QOL measures of bodily pain, mental health, and vitality improved on the SF-36.
Three studies (one Class I and two Class II) evaluated the efficacy of duloxetine in PDN.26–28 The Class I study showed that duloxetine had a small effect compared with placebo, reducing pain by 8% on the 11-point Likert scale26; QOL was not assessed. In two Class II studies, duloxetine reduced pain (measured by VAS) 13% more than placebo,27,28 but in one study a moderate effect was shown in the responder analysis, with 26% more responders on duloxetine 120 mg/day (total 52%) than placebo (26%) (responders defined as those patients having 50% reduction in their 24hour average pain score).27 The completion rate in both studies was about 75%.27,28 Duloxetine reduced interference with general activity and the SF-36 and Euro-QoL (EQ-5D).27,28
Three studies (one Class I and two Class II) evaluated the efficacy of amitriptyline.29–31 The Class I study showed a large responder effect with amitriptyline, with 43% more responders with amitriptyline than with placebo (requiring at least 20% pain reduction for responder status). A third group in this study that was treated with maprotiline had 18% more responders than the placebo group.29 In two Class II studies amitriptyline had a large effect, reducing pain on a verbal 13-item descriptor list converted to a numeric 5-point scale by 63% and 58%, respectively, more than placebo.30,31 In one of these Class II studies an active placebo was used.30
Two Class III trials evaluated other tricyclic antidepressants (imipramine and nortriptyline).32,33 One Class III study showed that 47% more subjects on imipramine improved on a global evaluation compared with the placebo group, but there was no difference on a 6-point symptom scale.32 Another Class III study showed a large effect with the combination of nortriptyline plus fluphenazine compared with placebo; 63% more patients had a 50% VAS reduction in the combination group.33 One Class III study compared desipramine, amitriptyline, fluoxetine, and placebo and found a small effect on a 13-word scale converted to 5 points for amitriptyline and desipramine (both had 6% pain reduction), but not for fluoxetine.34
Conclusions. Based on three Class I and five Class II studies, the antidepressants amitriptyline, venlafaxine, and duloxetine are probably effective in lessening the pain of PDN. Venlafaxine and duloxetine also improve QOL. Venlafaxine is superior to placebo in relieving pain when added to gabapentin. There is insufficient evidence to determine whether desipramine, imipramine, fluoxetine, or the combination of nortriptyline and fluphenazine are effective for the treatment of PDN. Recommendations:
| 1. | Amitriptyline, venlafaxine, and duloxetine should be considered for the treatment of PDN (Level B). Data are insufficient to recommend one of these agents over the others. |
| 2. | Venlafaxine may be added to gabapentin for a better response (Level C). |
| 3. | There is insufficient evidence to support or refute the use of desipramine, imipramine, fluoxetine, or the combination of nortriptyline and fluphenazine in the treatment of PDN (Level U). |
One Class I study showed that dextromethorphan relieved pain moderately by 16% more than placebo on a 20-point Gracely Box scale in PDN and improved the SF-36.35 In one Class II study, dextromethorphan with benztropine reduced pain by 24% more than placebo on a 6-point scale, a moderate reduction.36
A Class II study showed that morphine sulfate had a small effect and reduced pain from baseline by 15% on the SF-MPQ and improved the SF-36 and the Beck Depression Inventory.37
In two Class II studies, tramadol relieved pain moderately (16% and 20% more than placebo on a Likert scale) in PDN38,39 and improved physical function.38
In three Class II studies, oxycodone controlled release and Ultracet (tramadol þ acetaminophen) relieved pain in PDN.40e1,e2 Oxycodone had a small effect, with 9% more pain relief on the Pain Inventory than placebo. It also improved sleep quality by 7% more than placebo, but did not change SF-36.40 Ultracet improved pain by 13% on the VAS, a small effect, and also improved SF-36 scores by 10%.e1 Oxycodone controlled-release had a moderate effect on pain (27% reduction in the VAS compared with placebo), improved disability by 10%, and improved most SF-36 subscores.e2
Conclusions. Based on one Class I study, dextromethorphan is probably effective in lessening the pain of PDN and improving QOL. Based on Class II evidence, morphine sulfate, tramadol, and oxycodone controlled-release are probably effective in lessening the pain of PDN. Dextromethorphan, tramadol, and oxycodone controlled-release have moderate effect sizes, reducing pain by 27% compared with placebo.
Recommendations: Dextromethorphan, morphine sulfate, tramadol, and oxycodone should be considered for the treatment of PDN (Level B).
Data are insufficient to recommend one agent over the other.
Clinical Context. The use of opioids for chronic, nonmalignant pain has gained credence over the last decade due to the studies reviewed in this study. Both tramadol and dextromethorphan were associated with substantial adverse events (e.g., sedation in 18% on tramadol and 58% on dextromethorphan, nausea in 23% on tramadol, and constipation in 21% on tramadol). The use of opioids can be associated with the development of novel pain syndromes such as rebound headache. Chronic use of opioids leads to tolerance and frequent escalation of dose.
Other Pharmacological Agents. We identified 18 articles relevant to other pharmacological agents rated higher than Class IV (Supplementary table 4). Thirteen other articles were excluded. Most of the RCTs rated Class II instead of Class I had completion rates of <80%, and those rated Class III often lacked predefined endpoints.
One Class I study of 0.075% capsaicin showed a large effect, with 40% more pain reduction on the VAS compared with vehicle cream.e3 One Class II study showed that 0.075% capsaicin reduced pain in PDN with a small effect size of 13% on the VAS compared with vehicle cream.e4
One Class I study of isosorbide dinitrate spray showed a moderate effect, with an 18% reduction in VAS pain compared with placebo.e5
One Class I study of clonidine and pentoxifylline compared with placebo did not show an effect of these drugs on PDN.e6
One Class I study of mexiletine did not show an effect on PDN.e7 Two Class II studies showed pain reduction with mexiletine: one with a large effect (37% more pain reduction than placebo)e8 and one with a small effect (5% difference compared to placebo).e9 In the first Class II study, sleep disturbance was reduced,e8 but this was not found in the other Class II study.e9
In a single Class I study of sorbinil, pain relief was not observed.e10
One Class I and two Class II studies showed benefit from alpha-lipoic acid in reducing pain in PDN, but pain was not a predefined endpoint in these studies.e11–e13 The effect size in pain reduction was moderate (20–24% superior to placebo).
In two Class III studies, intravenous (IV) lidocaine decreased pain relative to placebo infusion.e14,e15 In one study, a transient decrease of 75% was observed on a 5-point symptom scale, compared with a decrease of 50% with placebo infusion.e14 In the other study, the McGill Pain Questionnaire improved by a small amount (9% reduction in present pain intensity) with lignocaine, and the differences with placebo were significant due to worsening in the placebo group.e15 The baseline values were not provided.
In two Class III studies, the lidoderm patch improved pain scores with a moderate to large effect (20–30% reduction in pain scores from baseline and 70% of patients experienced >30% relief in pain).e16,e17
Conclusions. Based on Class I and Class II evidence, capsaicin cream is probably effective in lessening the pain of PDN. Based on Class III studies, there is insufficient evidence to determine whether IV lidocaine is effective in lessening the pain of PDN. Based on Class III evidence, the lidoderm patch is possibly effective in lessening the pain of PDN. Based on Class I evidence, clonidine and pentoxifylline are probably not effective for the treatment of PDN. The evidence for the effectiveness of mexiletine is contradictory; however, the only Class I study of this agent indicates that mexiletine is probably ineffective for the treatment of PDN. There is insufficient evidence to determine whether vitamins and alpha-lipoic acid are effective for the treatment of PDN. Based on Class I evidence, isosorbide dinitrate spray is probably effective for the treatment of PDN. Recommendations:
| 1. | Capsaicin and isosorbide dinitrate spray should be considered for the treatment of PDN (Level B). |
| 2. | Clonidine, pentoxifylline, and mexiletine should probably not be considered for the treatment of PDN (Level B). |
| 3. | The lidoderm patch may be considered for the treatment of PDN (Level C). |
| 4. | There is insufficient evidence to support or refute the usefulness of vitamins and alphalipoic acid in the treatment of PDN (Level U). |
In patients with PDN, what is the efficacy of non-pharmacological modalities to reduce pain and improve physical function and QOL?
We identified 11 articles relevant to non-pharmacological treatment of PDN graded higher than Class IV (Supplementary table 5). Only articles on electrical stimulation, Reiki therapy, low-intensity laser therapy, and magnetized shoe insoles reached evidence levels sufficient for discussion in the text. Surgical decompression was addressed in a previous AAN practice advisorye18 and will not be considered further in this report.
Electrical Stimulation. One Class I study reported that percutaneous electrical nerve stimulation reduced pain in PDN by a large magnitude (42% on the VAS) compared with the reduction observed with sham treatment, and also improved sleep.e19 One Class II study reported no effect with electrical stimulation,e20 and one Class II study of frequency-modulated electromagnetic neural stimulation showed a small degree of pain relief (11% on the VAS) in a crossover design, but with no improvement in the placebo group.e21
The addition of electrotherapy to amitriptyline was more effective than amitriptyline alone, based on one Class III study.e22
Magnetic Field Treatment. One Class I study using pulsed electromagnetic fields compared with a sham device failed to demonstrate an effect in patients with PDN.e23
One Class II study of the use of magnetized shoe insoles in patients with PDN showed a small effect (14% VAS decrease) at 4 months compared with non-magnetized insoles, but the endpoint of burning pain was not predetermined.e24
Other Interventions. One Class I study on the use of low-intensity laser treatment compared with sham treatment did not show an effect on pain.e25
Reiki therapy is defined as the transfer of energy from the practitioner to the patient to enable the body to heal itself through balancing energy. One Class I study of Reiki therapy did not show any effect on PDN.e26
Other interventions such as exercise and acupuncture do not have any evidence of efficacy in treating PDN.
Conclusion. Based on a Class I study, electrical stimulation is probably effective in lessening the pain of PDN and improving QOL. Based on single Class I studies, electromagnetic field treatment, low-intensity laser treatment, and Reiki therapy are probably not effective for the treatment of PDN. There is not enough evidence to support or exclude a benefit of amitriptyline plus electrotherapy in treating PDN.
Recommendations:
| 1. | Percutaneous electrical nerve stimulation should be considered for the treatment of PDN (Level B). |
| 2. | Electromagnetic field treatment, low-intensity laser treatment, and Reiki therapy should probably not be considered for the treatment of PDN (Level B). |
| 3. | Evidence is insufficient to support or refute the use of amitriptyline plus electrotherapy for treatment of PDN (Level U). |
CLINICAL CONTEXT SUMMARY FOR ALL EVIDENCE
It is notable that the placebo effect varied from 0% to 50% pain reduction in these studies.
Adjuvant analgesic agents are drugs primarily developed for an indication other than treatment of PDN (e.g., anticonvulsants and antidepressants) that have been found to lessen pain when given to patients with PDN. Their use in the treatment of PDN is common.e33 The panel recognizes that PDN is a chronic disease and that there are no data on the efficacy of the chronic use of any treatment, as most trials have durations of 2–20 weeks. It is important to note that the evidence is limited, the degree of effectiveness can be minor, the side effects can be intolerable, the impact on improving physical function is limited, and the cost is high, particularly for novel agents.
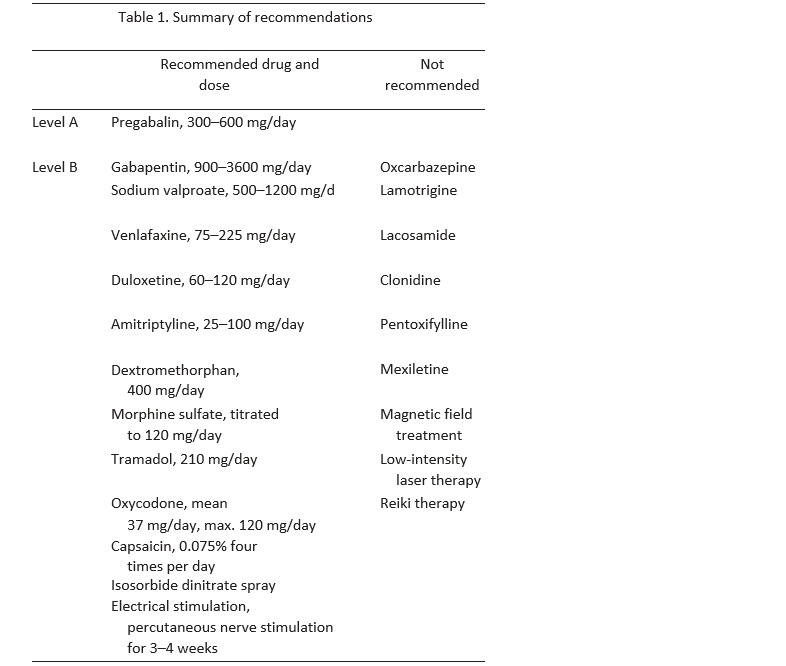
A summary of Level A and B recommendations for the treatment of PDN is provided in Table 1.
RECOMMENDATIONS FOR FUTURE RESEARCH
| 1. | A formalized process for rating pain scales for use in all clinical trials should be developed. |
| 2. | Clinical trials should be expanded to include effects on QOL and physical function when evaluating efficacy of new interventions for PDN and the measures should be standardized. |
| 3. | Future clinical trials should include head-to-head comparisons of different medications and combinations of medications. |
| 4. | Because PDN is a chronic disease, trials of longer duration should be done. |
| 5. | Standard metrics for side effects to qualify effect sizes of interventions need to be developed. |
| 6. | Cost-effectiveness studies of different treatments should be done. |
| 7. | The mechanism of action of electrical stimulation is unknown and a better understanding of its role, mode of application, and other aspects of its use should be studied. |
Search term used included painful diabetic neuropathy OR neuropathic pain OR diabetes AND: anticonvulsant, anti-epileptic, anti-depressant, antiarrthymic, spinal cord stimulation, infra-red therapy, acupuncture, opioids, topical patches, lidocaine, intra-thecal baclofen, TENS, vitamins, lifestyle modification, metabolic control, baclofen.
APPENDIX E2: AAN CLASSIFICATION OF EVIDENCE FOR RATING OF A THERAPEUTIC ARTICLE
Class I: A randomized, controlled clinical trial of the intervention of interest with masked or objective outcome assessment, in a representative population. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences. The following are also required:
| a. | Concealed allocation. |
| b. | Primary outcome(s) clearly defined. |
| c. | Exclusion/inclusion criteria clearly defined. |
| d. | Adequate accounting for dropouts (with at least 80% of enrolled subjects completing the study) and crossovers with numbers sufficiently low to have minimal potential for bias. |
| e. | For non-inferiority or equivalence trials claiming to prove efficacy for one or both drugs, the following are also required (note that 1–3 are required for Class II in equivalence trials. If any one of the three are missing, the class is automatically downgraded to Class III). |
| 1. | The authors explicitly state the clinically meaningful difference to be excluded by defining the threshold for equivalence or non-inferiority. |
| 2. | The standard treatment used in the study is substantially similar to that used in previous studies establishing efficacy of the standard treatment (e.g., for a drug, the mode of administration, dose, and dosage adjustments are similar to those previously shown to be effective). |
| 3. | The inclusion and exclusion criteria for patient selection and the outcomes of patients on the standard treatment are comparable to those of previous studies establishing efficacy of the standard treatment. |
| 4. | The interpretation of the results of the study is based upon a per-protocol analysis that takes into account dropouts or crossovers. |
Class III: All other controlled trials (including well-defined natural history controls or patients serving as own controls) in a representative population, where outcome is independently assessed, or independently derived by objective outcome measurement [i.e., an outcome measure that is unlikely to be affected by an observer’s (patient, treating physician, investigator) expectation or bias (e.g., blood tests, administrative outcome data)]. Class IV: Studies not meeting Class I, II, or III criteria including consensus or expert opinion.
APPENDIX E3: CLASSIFICATION OF RECOMMENDATIONS
| A | = | Established as effective, ineffective or harmful (or established as useful/predictive or not useful/ predictive) for the given condition in the specified population. (Level A rating requires at least two consistent Class I studies.) [In exceptional cases, one convincing Class I study may suffice for an ‘‘A’’ recommendation if: (1) all criteria are met; (2) the magnitude of effect is large (relative rate improved outcome >5 and the lower limit of the confidence interval is >2.] |
| B | = | Probably effective, ineffective, or harmful (or probably useful/predictive or not useful/predictive) for the given condition in the specified population. (Level B rating requires at least one Class I study or two consistent Class II studies.) |
| C | = | Possibly effective, ineffective, or harmful (or possibly useful/predictive or not useful/predictive) for the given condition in the specified population. (Level C rating requires at least one Class II study or two consistent Class III studies.) U ¼ Data inadequate or conflicting; given current knowledge, treatment (test, predictor) is unproven. |
References
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freedman R,et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962.
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The healthcare costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003;26:1790–1795.
- Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE,Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004;21:976–982.
- Farrar JT, Berlin JA, Strom BL. Clinically important changes in acutepain outcome measures: a validation study. J Pain Symptom Manage 2003;25:406–411.
- Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. Theclinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain 2010;11:109–118.
- Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinicalimportance of changes in chronic pain intensity measured on an 11point numerical pain rating scale. Pain 2001;94:149–158.
- Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relievessymptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 2004;63:2104–2110.
- Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, KnappLE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 2005;6:253–260.
- Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin forthe treatment of painful diabetic peripheral neuropathy: a doubleblind, placebo-controlled trial. Pain 2004;110:623–638.
- Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005;115:254–263.
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, HesM, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998;280:1831–1836.
- Gorson KC, Schott C, Herman R, Ropper AH, Rand WM. Gabapentin inthe treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial. J Neurol Neurosurg Psychiatry 1999;66:251–252.
- Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotriginereduces painful diabetic neuropathy: a randomized, controlled study. Neurology 2001;57:505–509.
- Vinik AI, Tuchman M, Safirstein B, Corder C, Kirby L, Quessy M,et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. Pain 2007;128:169–179.
- Kochar DK, Jain N, Agarwal RP, Srivastava T, Agarwal P, Gupta S. Sodium valproate in the management of painful neuropathy in type 2 diabetes—a randomized placebo controlled study. Acta Neurol Scand 2002;106:248–252.
- Kochar DK, Rawat N, Agrawal RP, Vyas A, Beniwal R, Kochar SK,et al. Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. QJM 2004;97:33–38.
- Raskin P, Donofrio PD, Rosenthal NR, Hewitt DJ, Jordan DM, XiangJ, et al. Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neurology 2004;63:865–873.
- Grosskopf J, Mazzola J, Wan Y, Hopwood M. A randomized, placebocontrolled study of oxcarbazepine in painful diabetic neuropathy. Acta Neurol Scand 2006;114:177–180.
- Dogra S, Beydoun S, Mazzola J, Hopwood M, Wan Y. Oxcarbazepinein painful diabetic neuropathy: a randomized, placebo-controlled study. Eur J Pain 2005;9:543–554.
- Beydoun A, Shaibani A, Hopwood M, Wan Y. Oxcarbazepine in painful diabetic neuropathy: results of a dose-ranging study. Acta Neurol Scand 2006;113:395–404.
- Rauck RL, Shaibani A, Biton V, Simpson J, Koch B. Lacosamide inpainful diabetic peripheral neuropathy: a phase 2 double-blind placebo-controlled study. Clin J Pain 2007;23:150–158.
- Wymer JP, Simpson J, Sen D, Bongardt S. Efficacy and safety oflacosamide in diabetic neuropathic pain: an 18-week double-blind placebo-controlled trial of fixed-dose regimens. Clin J Pain 2009; 25:376–385.
- Shaibani A, Biton V, Rauck R, Koch B, Simpson J. Long-term orallacosamide in painful diabetic neuropathy: a two-year open-label extension trial. Eur J Pain 2009;13:458–463.
- Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extendedrelease in the treatment of painful diabetic neuropathy: a doubleblind, placebo-controlled study. Pain 2004;110:697–706.
- Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J Clin Neuromuscul Dis 2001;3:53–62.
- Raskin J, Pritchett YL, Wang F, D’souza DN, Waninger AL, IyengarS, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6:346–356.
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs.placebo in patients with painful diabetic neuropathy. Pain 2005; 116:109–118.
- Wernicke JF, Pritchett YL, D’Souza DN, Waninger A, Tran P, IyengarS, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67:1411–1420.
- Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindstrom T, ThorellLH. A comparison of amitriptyline and maprotiline in the treatment of painful diabetic neuropathy in diabetics and nondiabetics. Clin J Pain 1997;13:313–323.
- Max MB. Endogenous monoamine analgesic systems: amitriptylinein painful diabetic neuropathy. Anesth Prog 1987;34:123–127.
- Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37: 589–596.
- Kvinesdal B, Molin J, Froland A, Gram LF. Imipramine treatment ofpainful diabetic neuropathy. JAMA 1984;251:1727–1730.
- Gomez-Perez FJ, Rull JA, Dies H, Rodriquez-Rivera JG, Gonzalez-Barranco J, Lozano-Castaneda O. Nortriptyline and fluphenazine in the symptomatic treatment of diabetic neuropathy. A double-blind crossover study. Pain 1985;23:395–400.
- Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effectsof desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250–1256.
- Sang CN, Booher S, Gilron I, Parada S, Max MB. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology 2002;96:1053–1061.
- Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-doseoral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology 1997;48:1212–1218.
- Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL.Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med 2005;352:1324–1334.
- Harati Y, Gooch C, Swenson M, Edelmen S, Greene D, Raskin P,et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998;50: 1842–1846.
- Sindrup SH, Andersen G, Madsen C, Smith T, Brosen K, Jensen TS.Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain 1999;83:85–90.
- Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodonefor pain in diabetiic neuropathy: a randomized controlled trial. Neurology 2003;60:927–934.
Document History
Accepted 28 February 2011
Copyright 2011 American Association of Neuromuscular and Electrodiagnostic Medicine.
Published online in Wiley Online Library (wileyonlinelibrary.com). DOI 10.1002/mus.22092
Reaffirmed by the Practice Issue Review Panel: 2021
Creation of New Guidelines, Consensus Statements, or Position Papers
AANEM members are encouraged to submit ideas for papers that can improve the understanding of the field. The AANEM will review nominated topics on the basis of the following criteria:- Members’ needs
- Prevalence of condition
- Health impact of condition for the individual and others
- Socioeconomic impact
- Extent of practice variation
- Quality of available evidence
- External constraints on practice
- Urgency for evaluation of new practice technology
