Consensus Statement: Diagnostic and Screening Laboratory Tests in the Assessment of Patients with Small Fiber Neuropathy (an Evidence-Based Review)
Report of the American Association of Neuromuscular & Electrodiagnostic Medicine Small Fiber Neuropathy Task Force
Hans D. Katzberg1, Yuen So2, Thomas Brannagan3, John England4, Mark Bromberg5, William David6, Pushpa Narayanaswami7
- University Health Network and University of Toronto, Toronto, Ontario, Canada
- Stanford Neuromuscular Research, Stanford University, Palo Alto, California, USA
- Columbia University Medical Center, New York, New York, USA
- Louisiana State University Health Sciences Center, New Orleans, Louisiana, USA
- University of Utah, Salt Lake City, Utah, USA
- Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA
- Beth Israel Deaconess Deaconess Medical Center/Harvard Medical School (P.N.), Boston, Massachusetts, USA
ABSTRACT
Small fiber neuropathy (SFN) presents with neuropathic pain, dysesthesia, and autonomic symptoms in the context of normal nerve conduction studies, necessitating specialized diagnostic approaches. This evidence-based review by the AANEM SFN Task Force evaluates the diagnostic utility of ancillary tests and appropriate screening laboratory investigations in the assessment of SFN. A comprehensive literature review was conducted using OVID MEDLINE and EMBASE from 1966 to January 2023. Studies were selected based on prespecified inclusion criteria requiring clinical symptoms consistent with SFN and normal large fiber conduction studies. Articles were independently reviewed and graded for evidence quality by multiple raters.
Thirteen diagnostic and two laboratory screening studies met criteria. Skin punch biopsy assessing intraepidermal nerve fiber density showed Class II evidence (sensitivity 74–78%, specificity 65–80%). Additional metrics included intraepidermal fiber length and inter-fiber distance. CCM showed potential (Class III) but lacked validation in isolated SFN. Indirect tests (laser/contact heat evoked potentials, cutaneous silent period) had variable sensitivity and high specificity. Lab screening identified metabolic/immune etiologies in up to 64%, though most evidence was Class III. Skin biopsy is the most validated direct diagnostic tool for SFN, though CCM and indirect assessments can aid in diagnosis. No test offers enough sensitivity or specificity to serve as a stand-alone gold standard. Despite limited high-level evidence, screening for metabolic and immune conditions may help identify etiologies. Standardized methods and population studies are needed to improve accuracy.
INTRODUCTION
Small caliber nerve fibers can be involved in polyneuropathies leading to symptoms of pain, temperature insensitivity and autonomic dysfunction. In some cases, there is exclusive dysfunction of the small fibers, a condition characterized as a small fiber neuropathy (SFN).
The diagnosis of polyneuropathy is based on a clinical neurological examination and routine nerve conduction studies, but when the latter is normal it can be difficult to document exclusive small fiber loss to confirm the diagnosis of a SFN (1). Quantitative sensory thresholds testing (QST) can be helpful to confirm abnormal heat and cold detection thresholds consistent with small fiber loss, but these are subjective tests which do not confirm localization to peripheral nerves (2). There are other ancillary tests available to diagnose SFN, including direct assessment of the small fibers in the skin by a punch biopsy (3). Although there is mounting experience with these techniques, the sensitivity and specificity of these studies are uncertain. Additional tests which can be helpful in the diagnosis of SFN include additional direct assessments such as corneal confocal microscopy and indirect electrophysiological, imaging and sudomotor tests (4). Additional limitations of these diagnostic tests also include the need for specialized equipment and variability in the quality of test results due to operator dependency.
Causes of SFN include definable acquired and inherited conditions, but many are often classified as idiopathic, making associations difficult. Published guidelines provide suggestions for the appropriate laboratory evaluation of patients with distal symmetric polyneuropathy (5). This evidence-based review aims to answer the question of appropriate testing for diagnostic confirmation of a SFN and to provide guidance for the additional screening of underlying comorbidities. Specifically, the two questions to be addressed are: 1) What are the diagnostic tests used to establish the diagnosis of SFN? 2) What are the appropriate screening laboratory tests in the diagnostic evaluation of patients with SFN?
Formation of the Expert Panel
The seven members of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) SFN Task Force with expertise in the assessment of patients with peripheral neuropathy including SFN participated in the review. In addition, all members have experience in evidence-based methodology and neuromuscular guideline and consensus statement development. Four members (YS, TB, WD, MB) serve on the AANEM Practice Issues Review Panel.
Description of the Analytic Process
Databases and dates searched included OVID MEDLINE (1966-Aug 2017), EMBASE (1980-Aug 2017 to Jan 2023) and search terms are detailed in the appendix. In the search, symptoms of a SFN were defined as burning and tingling in the extremities with clinical signs of deficits in pinprick or temperature sensation. Patients had to have normal nerve conduction studies (NCSs). Patients with normal NCSs but with clinical evidence of large fiber neuropathy were excluded. Articles were also excluded if small fiber dysfunction was thought to be part of the neuropathy phenotype but small fiber tests were not evaluated. Electrodiagnostic evaluation of patients with disorders of the autonomic nervous system including generalized or regional autonomic failure, orthostatic intolerance, neurodegenerative disorders and anhidrosis were not included and are reviewed elsewhere (6).
Articles were required to have a detailed description of the testing procedure by which the diagnosis of SFN was made. Articles which included patients presenting with painful feet were included if the clinical and electrophysiological requirements were described and fulfilled. Articles in which conditions were unclear as to the role of small fiber nerves in the underlying pathophysiology of the disease, such as fibromyalgia, were excluded. Quantitative sensory thresholds to pain and temperature were excluded as they are considered to be an extension of the physical examination and are reviewed elsewhere (2). Criteria for classification of evidence and corresponding recommendation levels were based on the American Academy of Neurology (AAN) evidence grading system. A summary of these classes, definitions, and key features is provided in Table 1.
Analysis of the Evidence
The PRISMA flow diagram of narrative review is shown in Figure 1. The search yielded 1950 references with abstracts. After reviewing abstracts, 281 full text articles were selected for additional review. Each full text article was reviewed by two raters (WD, MB, HK, YS, JE, TB) independently and then through additional reconciliation (PN, HK, WD) to select the final group of articles. Class IV articles were not included as these were unlikely to change the quality of recommendations. This strategy yielded a group of 13 diagnostic and 2 laboratory screening articles, selected for grading of evidence in the initial search.
Question 1: What are the diagnostic tests used to establish the diagnosis of sSFN?
Direct Small Fiber Assessment: Skin punch Biopsy and Corneal Confocal Microscopy
The superficial location of small unmyelinated and partially myelinated fibers in the skin offers a unique opportunity for direct visualization from biopsied tissue with limited sequelae. A 3- mm diameter disposable punch is used to access a superficial depth of 3-4 mm in the skin where epidermal nerve fibers can be assessed. While deeper biopsies were previously required to study innervation of sweat glands and hair follicles, more recent techniques have been able to evaluate sweat gland nerve fiber density using the standard 3-mm punch biopsy (7,8). By using neuronal specific ubiquitin hydrolase protein gene product 9.5 (PGP 9.5) immunostaining, small epidermal nerve fibers can be visualized and distinguished from non-neural structures on skin punch samples, allowing an assessment of both morphology and density. In contrast to the use of skin punch biopsy in the conventional assessment of dermatological conditions, the assessment of nerve structures requires: a) expertise applying standard counting rules for nerve fiber density and length as well as identifying morphological changes seen in small fiber neuropathies including axonal swellings, regeneration and branching and b) age and sex adjusted reference values used to determine if there is a reduction in fiber density at a standard location (usually 10 cm proximal to the ankle in the lateral leg) which can be used effectively for diagnosis. Normative values with 95% confidence intervals for intra-epidermal nerve fiber density have been established for the distal leg and the thigh (3).
Methodology, results and outcomes of articles evaluating direct assessment methods for diagnosing SFN are summarized in table 2. The current review identified four case-control studies examining the performance of skin punch biopsy (at a standard site 7-10 cm proximal to the lateral malleolus) in the diagnosis of SFN. Of note, although some centres use IENFD at proximal sites on the leg and in the upper extremity to calculate ratios to determine whether length-dependent or non-length dependent SFN is present, there were no such studies which met criteria for inclusion in this review. One study, evaluating intraepidermal nerve fiber density (IENFD) in 45 patients using 134 healthy controls, was graded as Class II with adequate blinding, completion rate and a broad spectrum of conditions leading to development of SFN (9). This study concluded that assessment of IENFD was a useful method to confirm the diagnosis of SFN, with a comparable diagnostic yield of 35/45 (78%) when using a receiver operating characteristic (ROC) analysis based 10.3 fibers/mm cut-off. This study acknowledged that the diagnostic yield depended on the approach used to estimate the reference values, as a lower diagnostic yield of 16/45 (35%) was found when using a fifth percentile control cut-off of 6.7 fibers / mm. Piscosquito et al. evaluated intraepidermal inter-fiber distance (IEIFD) in skin biopsies and compared this modality to healthy controls in an effort to identify SFN. This Class II study found that an IEIFD of 350 microns had a sensitivity of 74% and specificity of 94% in differentiating SFN compared to normal controls (10).
Two additional case control studies evaluating 52 patients with SFN were classified as Class III due to the narrow spectrum of patients studied, which in turn limited study generalizability (11,12). These studies described a 74%-75% sensitivity and 70%-80% specificity of IENFD when using cut-offs of 8.8 fibers/mm and a <5% of lower limit when using healthy control values. In addition to assessment of IENFD, Lauria et al. (11) evaluated and determined that intraepidermal nerve fiber length (IENFL) and intraepidermal nerve dermal area (IENDA) had similarly high sensitivities (76%-80%) in accurately identifying patients with SFN.
Corneal confocal microscopy (CCM) is an alternate method of assessing small fibers in vivo and does not require a biopsy. This method uses manual and automated protocols to measure corneal nerve fiber length (CNFL), corneal nerve branch density (CNBD) and corneal nerve fiber density (CNFD) as direct measures of small fiber morphology. A single Class III graded study by Perkins et al. of a cohort of patients with type 1 and 2 diabetes, with and without diabetic sensorimotor polyneuropathy (DSP), identified a lower threshold value of CNFD (below 8.6 mm/mm2 to rule in DSP and upper value of 15.3 mm/mm2 to rule out DSP) with 88% specificity and 88% sensitivity (13). Although this study, and other lower quality articles, have shown potential for CCM to serve as a measure of small fiber dysfunction in patients with DSP, there have been no similar studies evaluating CCM in a cohort of patients with confirmed SFN.
CONCLUSIONS
There were two Class II and two Class III studies demonstrating that reduced IENFD at a standard site 10 cm from the lateral malleolus can identify patients with SFN. In addition, one Class III study suggested that IENFL and IENDA could identify SFN, and one Class II study indicated that inter-fiber distance could do the same. None of the articles commented on the usefulness of IENFD at proximal thigh sites or on the utility of intraepidermal nerve fiber morphology in the assessment of SFN. A single Class III study addressed the effectiveness of evaluating CNFD on CCM for the diagnosis of SFN.
RECOMMENDATIONS
The evaluation of IENFD at the distal leg site is probably useful in identifying patients with SFN (Level B). Nerve fiber morphology, nerve fiber length and inter-fiber distance are possibly useful in identifying SFN (Level C). There is insufficient evidence to comment on the use of biopsy obtained from proximal thigh sites in the evaluation of SFN (Level U). Evaluating CNFD on CCM is possibly useful in the diagnosis of SFN (Level C).
Indirect Small Fiber Assessment: Electrophysiological, Imaging, and Sudomotor Tests
Methodology, results and outcomes of articles evaluating indirect assessment methods for diagnosing SFN are summarized in table 3. Electrophysiological tests include the sympathetic skin response (SSR), cutaneous silent period (CSP) assessment and electrochemical skin conductance (ESC), all of which record the stimulus-induced electrical activity of small unmyelinated and thinly myelinated fibers in the skin (14-16). The SSR procedure records electrodermal activity from the palm and sole after delivering a noxious (typically electrical) stimulation to the contralateral limb or trigeminal nerve, with responses usually occurring within 1200 ms in the upper extremities and 2400 ms in the lower extremities. One Class II case control study evaluated this technique along with other tests of small fiber function in 87 patients encompassing a broad group of patients with SFN and 174 healthy controls (17). The study used trigeminal stimulation and recorded the best latency and amplitude after three trial responses recording from the palms and soles. Using this methodology to detect SFN, a sensitivity of 33% (29/87 patients) and specificity of 77.6% was identified.
Lefaucher et al. also evaluated ESC, a method which that evaluates electrical conductance (in microsemens) simultaneously at the palms and soles by applying low-voltage current (<4 mV) over 2 minutes and determining the electrochemical reaction between sweat chloride ions and nickel plates (16). The study found limited sensitivity (49.4%) but high specificity (92.5%) for the diagnosis of SFN. An additional Class III study by Castro et al. evaluated ESC in 64 patients with transthyretin familial amyloid polyneuropathy (TTR-FAP) mutations experiencing grade 1 symptoms corresponding to small fiber sensory disturbance and 37 healthy controls (18). The optimal cut-off of the foot ESC measurements for the presence of symptoms was 74 microsemens (sensitivity = 67%; specificity = 76%), with an area under the ROC of 0.78.
The CSP test uses a brief suppression of voluntary contraction after strong stimulation of a cutaneous nerve, measuring prolongation in latency or reduction in amplitude as a measure of small fiber dysfunction (14). Two Class III case control trials studied the CSP in patients with SFN. One study included 30 healthy controls and 31 patients clinically suspected to have diabetic SFN (19) while the other enrolled 26 healthy controls and 26 patients with diabetic SFN (20). The first of these studies found that the latency parameter yielded a 32.6% sensitivity and 96.7% specificity for the diagnosis of SFN, while other showed a sensitivity of 77% with a specificity of 96.2%.
Evoked potentials can be used to assess small fiber dysfunction by using laser or contact heat stimuli to activate small caliber myelinated (A-delta) or unmyelinated (C) fibers while recording electroencephalographic responses. Contact heat evoked potentials (CHEPS) have an advantage over laser evoked potentials (LEP) in that temperature stimuli are easier to control, thus reducing burn risk (21). One Class III case control study, evaluating a narrow group of patients with idiopathic SFN and using CHEPS amplitude and latency as metrics, demonstrated a sensitivity of 69.1% and specificity of 87% in identifying SFN (22). LEP as a diagnostic test was evaluated in two Class III case control studies: a) Lefacheur et al. evaluated 87 patients and 174 controls and found a sensitivity of 64.4% and specificity of 87.4% (16) and b) Di Stefanoa et al. (23) evaluated 25 patients and 73 controls and found a similarly high sensitivity of 78% and specificity of 81%.
Laser doppler imaging (LDI) uses cutaneous contact stimulation heating and evaluates reflex-mediated neurogenic dilatation of the skin vasculature by an infrared camera in the stimulated area and has been used to identify small fiber dysfunction when an attenuated response is observed. The review identified a single Class III article (24) which used a LDI flare cutoff of <1.96 cm2 to identify a sensitivity and specificity of 54%.
Quantitative sensory axon reflex testing (QSART) works by electrically stimulating eccrine sweat glands via acetylcholine iontophoresis and evaluating the sweat production in adjacent skin, via an axon reflex (25). The volume of stimulus-induced sweat production is measured at four conventional sites in the distal upper and lower extremities. A single Class III case control study assessed QSART in 27 patients with diabetic SFN and compared the results to 61 healthy controls (20). The authors documented a 100% specificity and a 58% sensitivity. They also determined that combining the QSART test with CSP increased the sensitivity for identifying SFN to 77%. Colorimetric sudomotor adhesive pad testing is an alternate sudomotor test, in which sweat function is measured by an adhesive pad impregnated with blue cobalt II chloride solution which changes color from blue to pink after 10 minutes of contact with sweat (26). The pads can be applied to the feet and categorized for the amount of sweat reduction, which in turn indicates small fiber autonomic function. Datema et al. evaluated this test in 35 patients with SFN and compared them to 61 healthy controls and found a sensitivity of 29% and specificity of 93%. This Class III case control study demonstrated an improvement in the sensitivity to 71% and reduction in specificity to 67% by combining colorimetric sudomotor adhesive pad testing with assessment of skin wrinkling in response to water immersion (WISW) (27).
CONCLUSIONS
One Class II and one Class III study demonstrated that SSR and ESC tests have low to moderate sensitivity but adequate specificity in identifying patients with SFN. Two Class III studies evaluated CSP, showing high specificity but variable sensitivity for detecting SFN. High sensitivity and specificity were found in one Class III study evaluating CHEPS and two Class III studies evaluating LEP. LDI showed limited sensitivity and specificity for SFN according to a single Class III study. One Class III study each evaluating QSART and colorimetric sudomotor adhesive pad testing showed good specificity for detecting SFN, however, the sensitivity was limited unless CSP or WISW was added to each of these tests.
RECOMMENDATIONS
Indirect electrophysiological tests including CHEP and LEP are possibly effective in identifying patients with SFN (Level C). SSR, LDI, and ESC are possibly ineffective in identifying patients with SFN due to inadequate sensitivity (Level C) and CSP is possibly effective as a diagnostic test to confirm SFN (Level C). QSART and colorimetric sudomotor adhesive pad tests are ineffective as stand-alone tests for SFN diagnosis, however, sensitivity may be increased (rendering them possibly effective) by combining QSART with CSP and colorimetric sudomotor adhesive pad testing with WISW (Level C).
CLINICAL CONTEXT
The diagnosis of SFN remains primarily a clinical diagnosis focused on clinical symptoms and signs including neuropathic pain, dysesthesia, disruption of pain and temperature sensation in the context of normal large fiber sensory and motor function on examination and nerve conduction studies. Direct and indirect tests should be used as supportive in this diagnosis with the realization through this review of the limitations of ancillary tests and the absence of a reliable gold standard. One method to mitigate this challenge is the use of a combination of tests to confirm the diagnosis of SFN, with a greater number of abnormal tests including the testing of different aspects of small fiber function and integrity, including QST which is an additional method of functional test not covered in this review. This approach also provides a more comprehensive clinical picture that may reduce the chances of a single abnormal test leading to a false positive result, however, the effects on a true negative diagnostic yield with this approach should be considered. A clinical practice challenge with this approach includes the difficulty in reliably performing multiple diagnostic procedures at a high standard, which includes resource constraints and technical factors that limit availability of testing for many patients.
Question 2: What are the appropriate screening laboratory tests in the diagnostic evaluation of patients with SFN?
Level of Evidence:
There were no Class I or Class II studies that addressed the appropriate screening laboratory tests for patients with SFN. This is largely because most of the articles evaluating screening tests were conducted at specialized neuromuscular centers where SFN is assessed without appropriate controls. In addition, there have been no population-based testing or studies evaluating the yield of screening laboratory tests in an at-risk population to allow determination of the yield of these tests when evaluating SFN.
Table 4 summarizes the articles evaluating screening laboratory tests for SFN. Three Class III studies attempted to evaluate the various etiologies and laboratory tests used to determine appropriate diagnosis in patients with SFN (28-30). Although standardized testing was not performed in the reports, patients in these studies were investigated for toxic, metabolic, inflammatory, and infectious etiologies, which are known to be associated with polyneuropathy (Table 5. In these studies, no underlying cause for SFN was identified in 36%-77% of patients undergoing comprehensive testing. When evaluating those who were already known to have an underlying condition at screening, the studies identified additional comorbidities in 3%-27% of patients. (29,30).
De Greef et al demonstrated immunological conditions to be present in 19% of patients, with a specific SFN diagnosis identified via testing of angiotensin converting enzyme level (sarcoidosis, 3%), and antibodies to SSA/SSB (Sjogren’s disease, 1.3%) and anti-tissue transglutaminase (celiac disease 0.5%) (30). In this cohort, other non-specific autoimmune conditions testing positive for antibodies were present in 6.1% (ie positive antinuclear antibodies, anti-extractable nuclear antigen antibodies and anti-neutrophil cytoplasmic antibodies). Bitzi et al characterized 34.5% of patients as having an apparent autoimmune etiology, with abnormal tests comprising ESR, ANA, and SSA. Infectious etiologies, including hepatitis C virus (HCV) and HIV, were identified rarely in two of the studies (28,29). In the Gemignani study, Sjogren’s syndrome, HCV, rheumatoid arthritis, and mixed cryogloblulinemia were identified as causes of SFN. Another main category included metabolic abnormalities, such as B12 deficiency, diabetes, and impaired glucose tolerance in 14.3% (29), 12.1% (30) and 39% (28) of patients. Hemochromatosis identified via C282Y allele in 3 patients and monoclonal gammopathy of unknown significance identified by SPEP in 12 of 921 patients with SFN were felt to be either equal to or lower than the prevalence in the population and thus less likely associated with SFN (30).
One case control study (31) that evaluated novel antibodies including trisulfated heparin disaccharide (TS-HDS) and fibroblast growth factor receptor (FGFR-3) antibodies in patients with SFN was initially identified as a potential article for inclusion in this review but ultimately was excluded. Although a relatively high proportion of patients with SFN were identified as harboring one of the two antibodies in this study (33%), these antibodies have also been identified in a variety of other neuropathy subtypes. Thus, confirmation of these antibodies as causative in SFN is uncertain (32). Two articles (33,34) observed a high proportion (10-29%) of sodium channel gene variants (SCN9A, SCN10A, SCN11A) in small cohorts of patients with SFN but were ultimately excluded due to concerns about the clinical relevance of these mutations (35).
CONCLUSION
Multiple Class III studies show that testing for metabolic and immune conditions can identify new abnormalities in patients with SFN, even when an underlying condition is known. Specifically, tests of blood sugar including fasting blood sugar, oral 2-hour glucose tolerance testing (OGTT), HbgA1C and immune tests including ESR, TTG, ANA, SSA, SSB have been shown to be the most commonly abnormal. However, tests for immune dysfunction can often be nonspecific. Articles evaluating the use of screening for sodium channel pathogenic variants (SCN9A, SCN10A, SCN11A) or TS-HDS and FGFR-3 antibodies in patients with SFN were excluded due to the uncertain clinical relevance of the findings.
RECOMMENDATIONS
Laboratory testing is possibly helpful in patients with SFN, even when an existing condition is known that could explain the neuropathy (Level C). The highest yield tests are the 2-hour OGTT, HbgA1C, ESR, TTG, ANA, SSA, and SSB (Level C). There is insufficient evidence to evaluate the utility of screening for sodium channel mutations (SCN9A, SCN10A, SCN11A) or TS-HDS and FGFR-3 antibodies in patients with SFN (Level U).
CLINICAL CONTEXT
In the absence of high-level evidence pertaining to the appropriate screening tests in the evaluation of patients with SFN, it is recommended that clinicians apply a similar strategy to the testing pursued in patients with any generalized polyneuropathy. This includes evaluation for hyperglycemia with 2-hour OGTT and HgBA1C, B12 level, as well as serum protein electrophoresis with immunofixation as screening tests. As with cases of polyneuropathy, additional testing including systemic immune serology markers and genetic tests should be performed if there is a suspicion for these conditions based on the clinical presentation. To date there has been no convincing evidence that testing for sodium channel gene pathogenic variants or TS-HDS and FGFR-3 antibodies are helpful as screening laboratory tests in patients with isolated SFN.
RECOMMENDATIONS FOR RESEARCH
Additional research is recommended to standardize methodology and to evaluate the effects of age and sex on reference values for skin biopsy metrics in the evaluation of SFN. Additional studies are needed to confirm the diagnostic yield of indirect electrophysiological and sudomotor tests as well as noninvasive direct tests such as CCM in larger and broader groups of patients.
Larger population-based studies are recommended to determine the appropriate etiological tests to perform in patients with SFN.
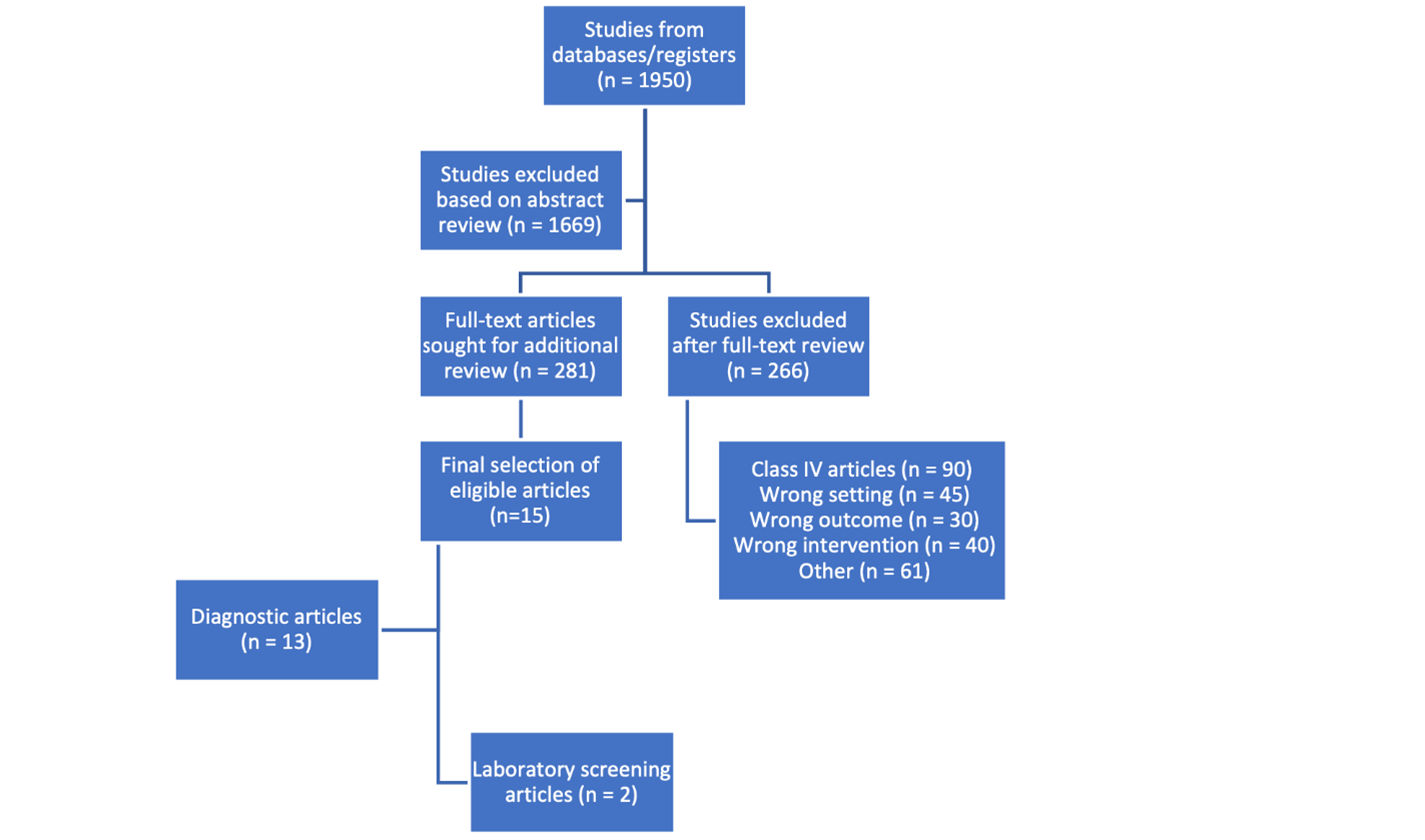
Table 1. Criteria for Classification of Evidence and Recommendation Levels
Class | Diagnostic Accuracy | Population Screening |
Class I | Cohort survey
with prospective data collection; includes a broad spectrum of individuals
suspected of having the disease; disease status determination is objective or
conducted blind to test result; plus: | Study of a
cohort at risk for the outcome from a defined geographic area
(population-based); outcome is objective; plus: |
Class II | Non–population-based, nonclinical cohort (e.g., volunteer panel) or general medical/neurology clinic without specialized interest. Meets criteria a & b from Class I; outcome is objective. | Non–population-based, nonclinical cohort or general clinic without specialized interest; meets criteria a & b from Class I; outcome is objective. |
Class III | Cohort or case-control study. | Referral cohort from a center with potential specialized interest in the outcome. |
Class IV | Studies not meeting Class I, II, or III criteria. | Studies not meeting Class I, II, or III criteria. |
Recommendation Level | Basis in Evidence | Interpretation |
Level A | Established as effective, ineffective, or harmful (requires at least 2 consistent Class I studies). | Strongest recommendation; high confidence in effect. |
Level B | Probably effective, ineffective, or harmful (requires at least 1 Class I study or 2 consistent Class II studies). | Moderate confidence in effect. |
Level C | Possibly effective, ineffective, or harmful (requires at least 1 Class II study or 2 consistent Class III studies). | Limited confidence in effect. |
Level U | Data inadequate or conflicting; treatment unproven. | No recommendation possible based on current evidence. |
Table 2. Direct Assessment for Small Fiber Neuropathy Assessment
Study | Class | Outcomes | Patients and Controls | Sensitivity | Specificity |
Nebuchennykh (9) | II | IENFD | 45 pts, 134 ctrl | 78% | 65% |
Piscosquito (10) | II | IEIFD | 31 pts, 31 ctrl | 74% | 94% |
Lauria (11) | III | IENFD | 25 pts, 25 ctrl | 75% | 80% |
|
| IENFL | 25 pts, 25 ctrl | 76% | 74% |
|
| IENDA | 25 pts, 25 ctrl | 80% | 78% |
Pourhamidi (12) | III | IENFD | 27 pts, 61 ctrl | 74% | 70% |
Perkins (13) | III | CCM | 998 pts, reference ctrl | 88% | 88% |
Legend: IENFD = intraepidermal nerve fiber density, IEIFD = intraepidermal inter-fiber distance, IENFL = intraepidermal nerve fiber length, IENFDA = intraepidermal nerve dermal area, CCM=corneal confocal microscopy (CCM), pts = patients, ctrl = controls
Table 3. Indirect Electrophysiological, Imaging and Sudomotor Small Fiber Tests
Study | Class | Outcomes | Patients and Controls | Sensitivity | Specificity |
Lefaucheur (17) | II | LEP, SSR, ESC | 87 pts, 174 ctrl | LEP 64.4%, SSR 33.3%, ESC 49.4% | LEP 87.4%, SSR 77.6%, ESC 92.5% |
Di Stefano (23) | III | LEP | 25 pts, 73 ctrl | 78% | 81% |
Lagerburg (22) | III | CHEPS | 42 pts, 97 ctrl | 69.1% | 87% |
Ebadi (24) | III | LDI | 26 pts, 48 ctrl | 54% | 54% |
Datema (27) | III | CSAPT | 35 pts, 61 ctrl | 29% | 93% |
Koytak (19) | III | CSP | 31 pts, 30 ctrl | 32.6% | 96.7% |
Kamel (20) | III | CSP, QSART | 27 pts, 26 ctrl | 77% CSP, 58% QSART | 96.2% CSP, 100% QSART |
Castro (18) | III | ESC | 64 pts, 37 ctrl | 67% | 76% |
Legend: LEP = laser evoked potentials, SSR = sympathetic skin response, ESC = electrochemical skin conductance, CHEPS = contact heat evoked potentials, LDI = laser doppler imaging, CSAPT= colorimetric sudomotor adhesive pad testing, CSP = cutaneous silent period, QSART = quantitative sensory axonal reflex test, pts = patients, ctrl = controls
Table 4. Screening Laboratory Tests for Small Fiber Neuropathy
Study | Class | Study type | Patients | Abnormal tests | Abnormal Tests |
Gemignani (28) | III | Case control | 44 | 22.7% | Presumed SSA, SSB, HCV, RF, OGTT, HgBA1C, cryoglobulins |
Bitzi (29) | III | Cohort | 84 | 64.3% | HgBA1C, ACE, HIV, HCV, ANA, ESR, SSA |
De Greef (30) | III | Cohort | 921 | 47% | TTG, 2-hour OGTT, B12, SPEP, SSA, SSB, CXR, SCN9A, SCN10 |
Legend: SCN = sodium channel voltage gated, TS-HDS = trisulfated heparin disaccharide, FGFR-3 = fibroblast growth factor receptor 3, OGTT = oral glucose tolerance test, SSA = Sjogren’s syndrome A , SSB = Sjogren’s syndrome B , HCV = hepatitis C virus, RF = rheumatoid factor, SPEP = serum protein electrophoresis, CXR = chest X-ray, HgBA1C = hemoglobin A1C, TTG = IgA anti-tissue transglutaminase
Table 5. Summary Screening Laboratory Small Fiber Tests
Category | Tests | Conditions/Details |
Metabolic Disorders | B12, B1, B6, folate, homocysteine, methylmalonic acid, fasting blood sugar, oral glucose tolerance test, hemoglobin A1c, liver enzymes, creatinine, thyroid hormones, cholesterol levels | Vitamin deficiency, diabetes mellitus, impaired glucose tolerance, hepatic disease, renal disease, thyroid dysfunction, hypercholesterolemia |
Hematological | Serum protein electrophoresis/immunofixation, cryoglobulins, C282Y allele testing | Monoclonal gammopathy of undetermined significance, neoplastic paraprotenemia, cryoglobulinemia, hemochromatosis |
Infections | HIV 1 and 2, Hepatitis B and C, Borrelia Burgdorferi IgI and IgM | HIV, hepatitis B and C, Lyme disease |
Systemic Immune Conditions | ANA, ANCA, C3/C4, DS-DNA, ENA, ESR, CRP, RF, ACE levels, IgA anti-tissue transglutaminase (TTG) | Sjogren syndrome, lupus, rheumatoid arthritis, sarcoidosis |
Genetic Factors | Sodium Channel Mutations (SCN4A, SCN9A), Fabry’s Disease, TTR amyloidosis | Isolated and systemic genetic conditions. |
Other Antibodies | TS-HDS, FGFR-3 | Of uncertain significance given lack of specificity |
Legend: ACE = angiotensin-converting enzyme; ANA = antinuclear antibody; ANCA = antineutrophil cytoplasmic antibody; B1 = thiamine; B6 = pyridoxine; B12 = cobalamin; CRP = C-reactive protein; C3/C4 = complement components 3 and 4; DS-DNA = double-stranded DNA antibody; ENA = extractable nuclear antigen; ESR = erythrocyte sedimentation rate; FGFR-3 = fibroblast growth factor receptor 3 antibody; HbA1c = hemoglobin A1c; HIV = human immunodeficiency virus; IgA = immunoglobulin A; IgM = immunoglobulin M; OGTT = oral glucose tolerance test; RF = rheumatoid factor; SCN4A/SCN9A = sodium channel, voltage-gated, type IV alpha subunit / type IX alpha subunit; SPEP = serum protein electrophoresis; TTR = transthyretin; TTG = tissue transglutaminase antibody; TS-HDS = trisulfated heparin disaccharide antibody.
References
- Saperstein DS. Small Fiber Neuropathy. Saperstein DS. Neurol Clin 2020;38(3):607-618.
- Chong PS, Cros DP. Technology literature review: quantitative sensory testing. Muscle Nerve. 2004;29(5):734-47.
- Engelstad JK, Taylor SW, Witt LV, Hoebing BJ, Herrmann DN, Dyck PJ, Klein CJ, Johnson DM, Davies JL, Carter RE, Dyck PJ. Epidermal nerve fibers: confidence intervals and continuous measures with nerve conduction. Neurology 2012; 79(22):2187-93.
- Verdugo RJ, Matamala JM, Inui K, Kakigi R, Valls-Solé J, Hansson P, Nilsen KB, Lombardi R, Lauria G, Petropoulos IN, Malik RA, Treede RD, Baumgärtner U, Jara PA, Campero M. Review of techniques useful for the assessment of sensory small fiber neuropathies: Report from an IFCN expert group. Clin Neurophysiol 2022;136:13-38.
- England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann DN, Howard JF, Lauria G, Miller RG, Polydefkis M, Sumner AJ; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2009 Jan;1(1):5-13.
- Cheshire WP, Freeman R, Gibbons CH, Cortelli P, Wenning GK, Hilz MJ, Spies JM, Lipp A, Sandroni P, Wada N, Mano A, Ah Kim H, Kimpinski K, Iodice V, Idiáquez J, Thaisetthawatkul P, Coon EA, Low PA, Singer W. Electrodiagnostic assessment of the autonomic nervous system: A consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol 2021 Feb;132(2):666-682.
- Mellgren SI, Nolano M, Sommer C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol 2013;115:171-88.
- Gibbons CH, Illigens BMW, Wang N, Freeman R. Quantification of sweat gland innervation. A clinical–pathologic correlation. Neurology 2009;72:1479–1486.
- Nebuchennykh M, Løseth S, Lindal S, Mellgren SI. The value of skin biopsy with recording of intraepidermal nervefiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J Neurol 2009 256:1067–1075.
- Piscosquito G, Provitera V, Mozzillo S, Caporaso G, Borreca I, Stancanelli A, Manganelli F, Santoro L, Nolano M. The analysis of epidermal nerve fibre spatial distribution improves the diagnostic yield of skin biopsy. Neuropathol Appl Neurobiol. 2021 Feb;47(2):210-217.
- Lauria G, Cazzato D, Porretta-Serapiglia C, Casanova-Molla J, Taiana M, Penza P, Lombardi R, Faber CG, Merkies ISJ. Morphometry of dermal nerve fibers in human skin. Neurology 2011;77:242–249.
- Pourhamidi K, Dahlin LB, Englund E, Rolandsson O. Evaluation of clinical tools and their diagnostic use in distal symmetric polyneuropathy. Primary Cre Diabetes. 2014;8:77-84.
- Perkins BA, Lovblom LE, Bril V, Scarr D, Ostrovski I, Orszag A, Edwards K, Pritchard N, Russell A, Dehghani C, Pacaud D, Romanchuk K, Mah JK, Jeziorska M, Marshall A, Shtein RM, Pop-Busui R, Lentz SI, Boulton AJM, Tavakoli M, Efron N, Malik RA. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologi. 2018;61(8):1856-1861.
- Floeter MK. Cutaneous silent periods. Muscle Nerve 2003;28:391–401.
- Novak P. Electrochemical skin conductance: a systematic review. Clin Auton Res 2019;29(1):17-29.
- Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res 2003;13:256–270.
- Lefaucheur JP, Wahab A, Planté-Bordeneuve V, Sène D, Ménard-Lefaucheur I, Rouie D, D. Tebbal D, Salhi H, Créange A, Zouari H, Ng Wing Tin S. Diagnosis of small fiber neuropathy: A comparative study of five neurophysiological tests. Clinical Neurophysiology 2015;45:445-455.
- Castro J, Miranda B, Castro I, de Carvalho M, Conceição I. The diagnostic accuracy of Sudoscan in transthyretin familial amyloid polyneuropathy. Clin Neurophysiol. 2016;127(5):2222-7.
- Koytak PK, Isak B, Borucu D, Uluic K, Tanridag T, Us O. Assessment of symptomatic diabetic patients with normal nerve conduction studies: utility of cutaneous silent periods and autonomic tests. Muscle Nerve 2011;43:317-323.
- Kamel JT. Vogrin SJ, Snight-Sadler RJ, Willems NK, Seiderer L, Cook MJ, Mac Isaac RJ, Roberts LJ. Combining cutaneous silent periods with quantitative sudomotor axon reflex testing in the assessment of diabetic small fiber neuropathy. Clinical Neurophysiology 2015;126):1047–1053.
- Y. Granovsky, P. Anand, A. Nakae, O. Nascimento, B. Smith, E. Sprecher, J. Valls-Solé. Normative data for Aδ contact heat evoked potentials in adult population: a multicenter study. Pain 2016; 157:1156-1163.
- Lagerberg V, Bakkers M, Bouwhuis A, Hoeijmakers J, Smit AS, Van Den Berg JM, Hordijk-de Boer I, Brouwer-Van Der Lee M, Dranendonk D, Reulen JP, Faber C, Merkies I. Contact Heat Evoked Potentials: Normal Values nd Use in Small-Fiber Neuropathy. Muscle Nerve 51:743-749.
- Di Stefano G, La CesaaS, Leonea C, Pepea A, Galosia E, Fiorellia M, Valerianib M, Lacerenzad M, Pergolinie M, Biasiottaa A, Cruccua G, Truinia A. Diagnostic accuracy of laser-evoked potentials in diabetic neuropathy. Pain. 2007;158:1100–1107.
- Ebadi H, Perkins BA, Katzberg MD, Lovblom LE, Bril V.Evaluation of Proxy Tests for SFSN: Evidence for Mixed Small and Large Fiber Dysfunction. PLoS ONE 7(8): e42208.23.
- Low PA, Caskey PE, Tuck RR, et al. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol 1983;14:573–80.
- Quattrini C, Jeziorska M, Tavakoli M, Begum P, Boulton AJM, Malik RA. The Neuropad test: a visual indicator test for human diabetic neuropathy. Diabetologia. 2008;51:1046–50.
- Datema Gert van Dijk MJ, Hoitsma E. The diagnostic value of water immersion skin wrinkling and Neuropads in small fiber neuropathy. Clinical Neurophysiology 123 (2012) 2074–2079.
- Gemignani F, Giovanelli M, Vitetta F, Santilli D, Bellanova MF, Brindani F, Barbin A. Non-length dependent small fiber neuropathy. A prospective case series. Journal of the Peripheral Nervous System 2010; 15:57–62.
- Bitzi LM, Lehnick D, Wilder-Smith EP. Small fiber neuropathy: Swiss cohort characterization. Ann Neurol. 2022 Jan;91(1):66-77.
- de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy - a large cohort study and review of the literature. Eur J Neurol. 2018 Feb;25(2):348-355.
- Zeidman LA, Kubicki K. Clinical Features and Treatment Response in Immune-Mediated Small Fiber Neuropathy with Trisulfated Heparin Disaccharide or Fibroblast Growth Factor Receptor 3 Antibodies. J Clin Neuromuscul Dis. 2021 Jun 1;22(4):192-199.
- Chompoopong P, Rezk M, Mirman I, Berini SE, Dyck PJB, Mauermann M, Shouman K, Klein C, Mills JR, Dubey D. TS-HDS autoantibody: clinical characterization and utility from real-world tertiary care center experience. J Neurol. 2023 Sep;270(9):4523-4528.
- Faber CG, Heijmakers J, Ahn H, Cheng X, Han C et al. Gain of Function NaV1.7 Mutations in Idiopathic Small Fiber Neuropathy. Ann Neurol 2012; 71:26-39.
- Huang J, Han C, Estacion M, Vasylyev D, Hoeijmakers J et al. Gain-of-function mutations in sodium channel NaV1.9 in painful neuropathy. Brain 2014; 137:1627-1942.
- Wadhawan S, Pant S, Golhar R, Kirov S, Thompson J, Jacobsen L, Qureshi I, Ajroud-Driss S, Freeman R, Simpson DM, Smith AG, Hoke A, Bristow LJ. NaVchannel variants in patients with painful and nonpainful peripheral neuropathy. Neurol Genet. 2017 Dec 15;3(6):e207.
Creation of New Guidelines, Consensus Statements, or Position Papers
AANEM members are encouraged to submit ideas for papers that can improve the understanding of the field. The AANEM will review nominated topics on the basis of the following criteria:- Members’ needs
- Prevalence of condition
- Health impact of condition for the individual and others
- Socioeconomic impact
- Extent of practice variation
- Quality of available evidence
- External constraints on practice
- Urgency for evaluation of new practice technology
